Macrophage
The main function of a macrophage is phagocytosis. On its surface there are many different receptors that are necessary for capturing viruses, fungi, bacteria, and immunoglobulins. Phagocytosis during proliferative inflammation may not always be complete, that is, it does not end with absolute digestion of the foreign agent. Viruses and microbial cells inside macrophages survive and multiply, causing the process to become chronic. In addition to macrophages, other cells are often found during proliferative inflammation. These include lymphocytes, eosinophils, plasma cells, mast cells, and single neutrophils.
With cell proliferation, cellular diffuse or focal infiltrates are formed.
Interstitial
Interstitial (or interstitial) is a type of proliferative inflammation in which a diffuse or focal cellular inflammatory infiltrate is formed in the stroma of the heart, liver, kidneys, and lungs. The infiltrate consists of lymphocytes, plasma cells, macrophages, eosinophils, single mast cells, elements of destroyed parenchyma, and rare neutrophils.
In the parenchymal elements, pronounced dystrophic, in some cases necrobiotic, changes are determined. The result of interstitial inflammation will be interstitial fibrosis, which is the proliferation of connective tissues.
How does proliferation occur?
Proliferation occurs at the very end of the inflammatory process, when destruction by bacteria and viruses that pathologically affect tissue ends. Signs of proliferation can be seen at the stage at which destroyed cells begin to regenerate, toxins begin to be eliminated, and damaged surface tissues begin to repair.
Of course, it is impossible to notice with a simple glance how inflammation replaces proliferation. All processes take place at the intracellular level. The b2-macroglobulin protein produced at this stage restores vascular permeability, decreased during the disease, and protects connective tissue from destruction. Free radicals disappear inside cells; they are neutralized by superoxide dismutase, a substance found in the human body, an antioxidant enzyme. At this stage proliferation occurs. That this is cellular rebirth is evident from the processes. Cells stop synthesizing pathogenic mediators, and new healthy receptors appear on their surface. The old ones are sucked inside and destroyed.
With polyps and genital warts
The proliferative phase of inflammation with the formation of polyps, as well as genital warts, is characterized by a chronic course. It is localized on the mucous membrane. On the mucous membranes of different organs, separate areas of hyperplasia are formed, as well as epithelial proliferation in the form of polyps, in which the connective tissue base is infiltrated with macrophages, lymphocytes, plasma cells and others.
It is most often localized on the mucous membrane of the nose, stomach, uterus, intestines, and bronchi. In the case of localization of inflammation at the junction of single-layer cylindrical and multilayer squamous epithelium, so-called condylomas are formed. These formations often appear in the anus, as well as on the genitals. In chronic proliferative inflammation, common condyloma is genital, which is caused by papillomavirus. It is considered a risk factor for the development of squamous cell carcinoma.
Cell proliferation and cancer
Hormones are regulators of cell growth. Peptide hormones such as GH, IGF-1 and IGF-2 stimulate linear body growth and cell proliferation in many tissues (Chapter 7). Other peptides [fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), TGF-a and TGF-JJ] have a similar effect. Triple factors regulate the growth of endocrine glands. Thus, ACTH and angiotensin II affect the size of the adrenal glands, TSH - the size of the thyroid gland, and LH and FSH - the size of the ovaries and testes. Thyroid hormones stimulate the growth of many tissues. Steroid hormones can either stimulate or inhibit cell growth. Glucocorticoids inhibit the proliferation of some cells and cause the death of lymphocytes, while estradiol, testosterone and dihydrotestosterone stimulate the growth of the mammary glands and prostate gland, respectively.
Hormones influence the growth of cancer cells, often in the same way as normal, undifferentiated cells. An example is the effect of estrogens on breast cancer and the effect of androgens on prostate cancer. However, cancer can also be caused by disruption of hormonal signaling pathways. As noted above, many growth factor analogues and their receptors are encoded by oncogenes.
Granulomatous
Granulomatous is another variant of productive (proliferative) inflammation. During which the main morphological substrate is considered to be a granuloma, where cells predominate: macrophages, as well as their derivatives (giant cells, epithelioid).
The morphogenesis of granulomas has four successive phases. These include the following:
- accumulation of young monocytes at the site of damage;
- maturation of these cells in a macrophage with the formation of a macrophage granuloma;
- further maturation and transformation of monocytes and macrophages into an epithelioid cell and the formation of an epithelioid cell granuloma;
- transformation of an epithelioid cell into a Pirogov-Langhans giant cell (foreign body cell) and the formation of giant cell granulomas.
It should be noted that the phagocytic activity of the granuloma cell gradually decreases as it matures.
The diameter of granulomas is about 1-2 mm, most often they are visible only under a microscope. In the central region of the granuloma, tissue detritus can be seen, which is formed as a result of tissue necrosis and in which the causative agent of the underlying disease can be detected, if in this case there is an infectious process. Macrophages are located along the periphery of necrosis. There are also giant, epithelioid cells, among them there may also be plasma cells, neutrophils, lymphocytes, and eosinophils.
Granulomatous diseases
Among such diseases in the form of proliferative inflammation, 4 groups are distinguished. These include:
- infectious etiology, which should include rheumatism, typhus and typhoid fever, rabies, brucellosis, tularemia, viral encephalitis, yersineosis, actinomycosis, syphilis, leprosy, schistosomiasis, tuberculosis, scleroma, glanders and others;
- non-infectious etiology, which includes gout, silicosis, anthracosis, talcosis, asbestosis, berylliosis, aluminosis;
- drug-induced illnesses, for example, drug-induced hepatitis, oleogranulomatous disease;
- diseases of unknown etiology: Crohn's disease, sarcoidosis, Horton's disease, Wegener's granulomatosis, rheumatoid arthritis, xanthogranulomatous pyelonephritis.
Absolutely all granulomas have an infectious etiology; despite the differences, they are similar in morphology. It is also worth noting that in all situations, infectious granulomas appear in the form of clusters of cells of a monocyte-macrophage nature. In some granulomas, lymphocytes, neutrophils, and plasma cells are formed; with helminthiasis, many eosinophils appear.
The only exceptions will be granulomas in the case of tuberculosis, syphilis, scleroma, glanders, and leprosy. In these diseases with proliferative inflammation, these granulomas have specific features that are characteristic only of a specific pathogen. And this allows us to classify this group of diseases as specific granulomatosis. Or specific inflammation.
In the morphological concept, specific inflammation will be characterized by the formation of several specific granulomas. Which have a characteristic structure. It may differ depending on the main pathogen - the cause of proliferative inflammation. Thus, the cellular composition, as well as the location of the cells directly in the granuloma, is quite specific for each pathogen.
Home / Publisher / Mechanisms of development of proliferation in the focus of inflammation
2.1.10. Mechanisms of proliferation development at the site of inflammation
Proliferation is the final phase of the development of inflammation, ensuring reparative tissue regeneration at the site of the source of inflammation.
iterations. Proliferation develops from the very beginning of inflammation along with the phenomena of alteration and exudation.
During reparative processes at the site of inflammation, cell regeneration and fibroplasia are achieved both by activating proliferation processes and by limiting cell apoptosis. The proliferation of cellular elements begins along the periphery of the inflammation zone, while in the center of the lesion the phenomena of alteration and necrosis can still progress. Proliferation of connective tissue and organ-specific cellular elements reaches full development after “cleansing” the damaged area of cellular detritus and infectious agents of inflammation by tissue macrophages and neutrophils. In this regard, it should be noted that the process of proliferation is preceded by the formation of neutrophil and monocyte barriers, which are formed along the periphery of the alteration zone.
The restoration and replacement of damaged tissues begins with the release of fibrinogen molecules from the vessels and the formation of fibrin, which forms a kind of mesh, a framework for subsequent cell reproduction. Already along this framework, rapidly formed fibroblasts are distributed in the repair site. The division, growth and movement of fibroblasts are possible only after they are bound to fibrin or collagen fibers. This connection is provided by a special protein – fibronectin. The proliferation of fibroblasts begins along the periphery of the inflammation zone, ensuring the formation of a fibroblastic barrier. Chemotaxis, activation and proliferation of fibroblasts are carried out under the influence of:
1. Fibroblast growth factors.
2. Platelet-derived growth factor.
3. Cytokines - TNF, IL-1.
4. Kininov.
5. Thrombina.
6. Transforming growth factor b.
At first, fibroblasts are not mature and do not have sufficient synthetic activity. Maturation is preceded by internal structural and functional restructuring of fibroblasts: hypertrophy of the nucleus and nucleolus, hyperplasia of the ER, increased content of enzymes, especially alkaline phosphatase, nonspecific esterase, b-glucuronidase. Only after restructuring, fibroblasts begin to synthesize collagen, elastin, collagen-associated proteins and proteoglycans. Collagenogenesis is stimulated by the following biologically active substances - TNF, IL-1, IL-4, fibroblast growth factor, platelet-derived growth factor.
Intensively multiplying fibroblasts produce acidic mucopolysaccharides - the main component of the intercellular substance of connective tissue (hyaluronic acid, chondroitinsulfuric acid, glucosamine, galactosamine). In this case, the inflammation zone is not only encapsulated, but also a gradual migration of cellular and acellular components of the connective tissue from the periphery to the center begins, the formation of a connective tissue skeleton at the site of primary and secondary alteration.
Along with fibroblasts, other tissue and hematogenous cells also multiply. When the basement membranes of blood vessels are destroyed in the alteration zone, endothelial cells migrate along a gradient of angiogenic factors. The lumen of the newly formed capillary is formed by the fusion of the extracellular spaces of neighboring endothelial cells. Around the newly formed capillaries, mast cells, macrophages, and neutrophils are concentrated, which release biologically active substances that promote the proliferation of capillaries.
The most important factors stimulating angiogenesis are:
1. Fibroblast growth factors (basic and acidic).
2. Vascular endothelial growth factor.
3. Transforming growth factors .
4. Epidermal growth factor.
Fibroblasts, together with newly formed vessels, create granulation tissue. This is essentially young connective tissue, rich in cells and thin-walled capillaries, the loops of which protrude above the surface of the tissue in the form of granules.
The main functions of granulation tissue are: protective - preventing the influence of environmental factors on the source of inflammation and reparative - filling the defect and restoring the anatomical and functional usefulness of damaged tissues.
The formation of granulation tissue is not strictly necessary. This depends on the size and depth of the damage. Granulation tissue usually does not develop during the healing of bruised skin wounds or minor damage to the mucous membrane (Kuzin M.I., Kostyuchenok B.M. et al., 1990). Granulation tissue gradually turns into fibrous tissue called scar. In scar tissue, the number of vessels decreases, they become empty, the number of macrophages and mast cells decreases, and the activity of fibroblasts decreases. A small part of the cellular elements, located among the collagen filaments, remains active. It is assumed that tissue macrophages that remain active take part in the resorption of scar tissue and ensure the formation of softer scars.
In parallel with the maturation of granulations, epithelization of the wound occurs. It begins in the first hours after damage, and within the first day 2-4 layers of basal epithelial cells are formed. The rate of epithelialization is ensured by the following processes: migration, division and differentiation of cells. Epithelization of small wounds is carried out mainly due to the migration of cells from the basal layer. Larger wounds are epithelialized due to migration and mitotic division of cells of the basal layer, as well as differentiation of the regenerating epidermis. The new epithelium forms the boundary between the damaged and underlying layers; it prevents dehydration of wound tissue, a decrease in electrolytes and proteins in it, and also prevents the invasion of microorganisms.
Organ-specific cellular elements of organs and tissues also participate in the process of proliferation. From the point of view of the possibilities of proliferation of organ-specific cellular elements, all organs and tissues can be classified into three groups:
The first group may include organs and tissues whose cellular elements have active or practically unlimited proliferation, sufficient to completely compensate for the structural defect in the area of inflammation (epithelium of the skin, mucous membranes of the respiratory tract, gastrointestinal mucosa, genitourinary system; hematopoietic tissue, etc.).
The second group includes tissues with limited regenerative abilities (tendons, cartilage, ligaments, bone tissue, peripheral nerve fibers).
The third group includes those organs and tissues where organ-specific cellular elements are not capable of proliferation (heart muscle, CNS cells). The main factors regulating the processes of proliferation and differentiation of cells in the area of inflammation are:
1. Growth factors produced by macrophages, lymphocytes, platelets, fibroblasts and other cells stimulated in the inflammatory zone. These include:
— epidermal growth factors (stimulator of proliferation and maturation of the epithelium, stimulator of angiogenesis);
— transforming growth factor- (stimulator of angiogenesis);
— transforming growth factor- (fibroblast chemoattractant, stimulator of collagen, fibronectin, angiogenesis synthesis, proteolysis inhibitor);
— platelet-derived growth factor (stimulator of migration, proliferation and protein synthesis in target cells, has a pro-inflammatory effect);
— endothelial cell growth factor;
— acidic and basic fibroblast growth factor (stimulators of proliferation of all cells of the vascular wall);
— colony-stimulating factors (granulocyte and macrophage stimulators of differentiation, proliferation and functional activity of granulocyte and monocyte cells) — cytokines (TNF, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6 , IL-7), produced by T- and B-lymphocytes, mononuclear cells, mast cells, fibroblasts, endotheliocytes, provide chemotaxis, fibrogenesis, inhibit apoptosis, stimulate proliferation processes in the inflammation site. Growth inhibitors for some cells are the same cytokines that stimulate the proliferation of others - these are TNF, transforming growth factor and -interferon (Zaichik A.Sh., Churilov L.P., 1999);
— nerve growth factor (stimulator of proliferation, growth, morphogenesis of sympathetic neurons, epithelial cells). Growth factors, interacting with receptors on target cells, can directly stimulate DNA synthesis in cells or prepare intracellular receptors and enzymes for mitotic activity.
2. Calcitonin-related gene peptide stimulates the proliferation of endothelial cells, and substance P induces the production of TNF in macrophages.
3. Group E prostaglandins potentiate regeneration by increasing blood supply.
4. Keylons and antikeylons produced by various cells, acting on the feedback principle, can activate and inhibit mitotic processes in the focus of inflammation (Bala Yu.M., Lifshits V.M., Sidelnikova V.I., 1988) .
5. Polyamines (putrescine, spermidine, spermine), found in all mammalian cells, are vital for cell growth and division.
They provide stabilization of plasma membranes and the superhelical structure of DNA, protection of DNA from the action of nucleases, stimulation of transcription, methylation of RNA and its binding to ribosomes, activation of DNA ligases, endonucleases, protein kinases and many other cellular processes. Enhanced synthesis of polyamines, promoting proliferative processes, is noted in the focus of alteration (Berezov T.T., Fedoronchuk T.V., 1997).
6. Cyclic nucleotides: cAMP inhibits, and cGMP activates proliferation processes.
Wound healing.
Morphologically, the process of wound healing can proceed differently, depending on the anatomical substrate of the lesion, the degree of infection, the general condition of the body, and the nature of therapeutic measures (Kuzin M.I., Kostyuchenok B.M. et al., 1990). However, in any case, the course of the wound process reflects one of the classical types of healing:
1. Healing by primary intention.
2. Healing by secondary intention.
3. Healing under the scab.
Healing by first intention. This type of healing is characterized by fusion of the wound edges without visible intermediate tissue, through the connective tissue organization of the wound canal. Healing by primary intention is the most economical type of healing. For healing by primary intention, the following conditions are necessary:
1. Small damage area.
2. Tight contact of the wound edges.
3. Preservation of the viability of the wound edges.
4. Absence of foci of necrosis and hematoma.
5. Asepticity of the wound.
The morphological picture of healing by primary intention is manifested by moderate hyperemia, tissue edema in the wound walls, proliferation of fibroblasts and new formation of capillaries through endothelialization of channels and cracks in thickening fibrin (autochthonous mechanism) from one edge of the wound to the opposite. On the 6-8th day, granulation tissue firmly connects the walls of the wound, and during this period epithelization stops. In surgical practice, healing by primary intention is possible in two cases: with small wound sizes (the edges are no more than 10 mm apart from each other), as well as with surgical interventions that end with suturing. Local changes in the wound area are slightly expressed (swelling of the edges, hyperemia, infiltration, pain). General manifestations include increased body temperature, which gradually decreases by the 3rd day after surgery. Changes in the morphological composition of the blood are insignificant or absent. Neutrophilic leukocytosis and an increase in ESR up to 20 mm/h are sometimes observed. On the 5-6th day, these indicators usually normalize.
Wound healing by secondary intention occurs with extensive tissue damage, with the presence of non-viable tissue in the wound, hematoma, and with the development of infection in the wound. Any of these factors leads to healing by secondary intention. With various options for the course of healing by secondary intention, we are talking about the healing of a purulent wound, that is, healing through suppuration and granulation. On the 5-6th day after the alteration, after the rejection of necrotic cells, islands of granulation appear in the wound, which, gradually growing, fill the entire wound cavity. Changes in the nature of granulations always objectively reflect complications of healing that can occur under the influence of local and general factors. Reorganization of the scar is manifested by active epithelialization of the wound. The epithelium grows on the surface of the granulations in the form of a bluish-white border very slowly. In addition to epithelization, healing is facilitated by the phenomenon of wound contraction - uniform concentric contraction of the edges and walls of the wound. This phenomenon is explained by the appearance in the granulation tissue during the period of regeneration of fibroblasts with the ability to contract.
Healing of a wound under a scab is typical for minor injuries (abrasions, scratches, small area burns of the 1st and 2nd degree). The wound process begins with the coagulation of spilled blood or only lymph, which dries to form a scab. Underneath it, rapid regeneration of the epidermis occurs, and the scab is then rejected. The whole process lasts 3-7 days. If healing under the scab occurs without complications, then the wound heals by primary intention; if suppuration begins under the scab, then healing proceeds by secondary intention. In some cases, a sluggish phlegmonous lesion of the fatty tissue surrounding the wound may develop. In such a situation, surgical treatment of the wound and removal of the scab are necessary (Kuzin M.I., Kostyuchenok B.M. et al., 1990).
previous section | content| next section
For tuberculosis
The inflammatory process in tuberculosis, that is, Mycobacterium tuberculosis, can cause three types of tissue reactions: exudative, alterative, and proliferative.
As for alterative inflammation, it often develops as a result of hypoergy, in the event of a decrease in the protective forces of the human body. This inflammation manifests itself morphologically as caseous necrosis.
The exudative type of inflammation develops as a result of existing hyperergy (in case of increased sensitivity to mycobacterial toxins, antigens). Morphologically, accumulation manifests itself in the lesion of fibrinous, serous or mixed exudate, which later also undergoes caseous necrosis.
Proliferative inflammation, pathological anatomy says, develops in the condition of a specific tuberculous immune system. The morphological manifestation in this case will be the formation of so-called tuberculous granulomas, presented in the form of millet grains.
Water-salt metabolism
Hormones regulate many aspects of water-salt metabolism. Thus, vasopressin changes serum osmolality and water excretion (Chapter 6) and affects many functions of the cardiovascular system and the central nervous system. The mineralocorticoid aldosterone regulates the ratio of sodium and potassium ions and, to some extent, the ratio of chloride and bicarbonate ions. Other hormones also take part in the regulation of ion balance: ANP, insulin, glucagon, catecholamines, angiotensin II and PTH.
Next:
- Advances of modern science and endocrinology. 1 part
- Bone. Reproductive system. The immune system. central nervous system
- Intermediate exchange. Water-salt exchange. Cardiovascular system and kidneys
Tuberculous granuloma
So, we have looked at what characterizes proliferative inflammation. Now it is worth considering separately some cases in which it manifests itself.
Tuberculous granuloma has a characteristic structure: in its central region there is a focus of so-called caseous necrosis, behind which there is a shaft of radially localized (that is, elongated to the periphery from the center) epithelioid cells. Behind these cells, giant single Pirogov-Langhans cells are visible.
It should also be noted that at the periphery of such a granuloma there is a shaft of lymphocytes. In a large number of these typical cells, plasma cells and macrophages can also be found in small numbers. In addition, a thin network consisting of argyrophilic fibers is also revealed here. As for blood vessels, they are not found here. In the case of Ziehl-Neelsen staining, Mycobacterium tuberculosis can be identified in these giant cells.
Intermediate exchange
Insulin, glucagon, somatostatin, GH, catecholamines (adrenaline, norepinephrine), thyroid hormones, glucocorticoids and other hormones regulate the metabolism of carbohydrates, fats, proteins, amino acids and nucleic acids. In the regulation of intermediate metabolism, hormones act in a coordinated manner, adapting the body to various living conditions, such as stress or starvation. Insulin lowers blood glucose levels and stimulates the synthesis of fat, proteins and nucleic acids, while cortisol, glucagon, catecholamines and GH, on the contrary, act in various ways to increase blood glucose levels. These hormones affect the metabolism of proteins, fats and nucleic acids in different ways. Hormones alter the uptake of glucose, amino acids, nucleosides, and other small molecules into cells. For example, insulin enhances glucose uptake by causing glucose transporters to move to the plasma membrane. Hormones also influence the quantity and activity of enzymes involved in intermediate metabolism - gluconeogenesis, lipolysis, glycogen synthesis, metabolism and synthesis of amino acids, lipid synthesis, etc.
Inflammatory process in syphilis
The inflammatory process during syphilis in different periods will reflect different tissue reactions to treponema pallidum: as a rule, primary, secondary, and tertiary periods are distinguished in case of syphilis.
In the case of primary syphilis, a so-called productive-infiltrative reaction develops in the area of treponema introduction.
During the secondary, a strongly expressed exudative reaction is observed, promoting the generalization of the pathogen,
In the case of the tertiary period of syphilis, the productive-necrotic reaction will be presented in the form of a syphilitic granuloma, as well as gummous infiltrates.
More about syphilitic granuloma
Syphilitic granuloma in the field of medicine also goes by the short name “gumma”. In this granuloma, as in the case of tuberculosis, caseous necrosis is found in the center, but in this situation it will be larger in size.
From necrosis, a large number of lymphocytes, fibroblasts, and plasma cells are located along the periphery. Macrophages, giant cells, and epithelioid cells may be present in small numbers. What is characteristic in this case is the proliferation of connective tissues (this is due to the rapid proliferation of fibroblasts), which form something like capsules, as well as a large number of blood vessels.
Quite rarely, among these cells, specialists are able to identify the so-called Treponema pallidum using the Levaditi silvering method. Gumma is characteristic of the tertiary period of syphilis, which begins to develop several years (5 or more) from the time of infection.
In various organs: skin, liver, bones, brain, nodes measuring 0.3-1.0 cm in diameter are formed within one decade. In cross-section, a certain jelly-like mass of a yellowish tint stands out from these nodes, which in its appearance resembles gum arabic glue, which is where the name “gum” comes from.
Proliferation in gynecology
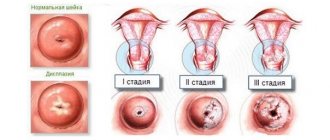
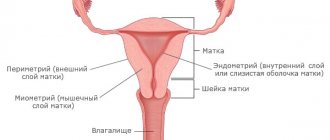
In the life cycle of a woman of childbearing age, proliferation occurs regularly. During menstruation, the endometrium is shed and then restored. Therefore, when taking hysteroscopy - scraping from the wall of the uterus - or when examining with an ultrasound machine, it is very important to take into account what phase of endometrial proliferation. During the monthly cycle, the endometrium has different thickness, and it is by this that the functioning of a woman’s reproductive organs is judged.
The growth phase of the endometrium is a very important parameter for assessing the pathomorphological picture. Without knowing this parameter, it is impossible to make an accurate diagnosis even for an experienced specialist.
Gummous infiltration
In addition to these gummas, gummatous infiltration can also develop in the tertiary period of syphilis disease. The infiltrate is represented by the same cells, that is, sclerosis, vascular proliferation. The infiltrate is most often localized in the ascending part of the heart, as well as in the aortic arch, and is called “syphilitic mesoaortitis”.
It, located in the central and outer shell of the cardiac aorta, gradually destroys its elastic frame, and connective tissue begins to grow in place of the elastic fibers. Because of all this, the inner lining of the aorta becomes uneven and wrinkled with a large number of scar retractions, bulges, outwardly resembling shagreen skin.