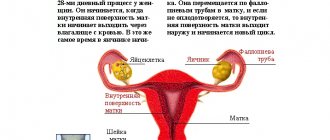
Ovarian hypofunction is a set of pathological changes in the female body,
as a result of which disorders of the menstrual cycle and the functioning of the reproductive system as a whole develop.
Ovarian failure rarely occurs in isolation. This pathology is observed infrequently and affects about 0.1% of women of fertile age and about 1% of the fairer sex after 40 years.
Much more often hypofunction is observed in patients with secondary amenorrhea. Despite the fact that the pathology does not pose an immediate danger to the patient, it causes a whole range of psychosomatic and vegetative manifestations that significantly reduce the standard of living.
What is ovarian hypofunction called?
Ovarian hypofunction is a pathological decrease in the functional activity of the female gonads. This condition leads to disturbances in the ovarian-menstrual cycle and related disorders, which becomes a reason to consult a doctor and undergo an examination.
This condition should not be confused with the physiological decline of reproductive function in the premenopausal period. Moderate ovarian hypofunction in women after 40 years of age (compared to their activity at 25-30 years of age) is normal. It is associated with the natural depletion of ovarian reserve and a physiological decrease in the activity of the hypothalamic-pituitary-ovarian system. If this is not accompanied by early cessation of menstruation and the appearance of other complaints, there is no need to worry. In this case, doctors will only diagnose the onset of the premenopausal period, which during its normal course does not require any treatment.
Ovarian hypofunction can be primary or secondary. This division is based on the level of the primary lesion. If the cause of the syndrome is damage to the ovarian tissue itself, they speak of primary hypofunction. And in case of disturbances at any higher level of neuroendocrine regulation of gonadal function, the secondary nature of the disorder is indicated.
Prevention of ovarian failure
Preventive measures only make sense if we are talking about secondary ovarian failure. It is important to follow a few simple recommendations:
- It is worthwhile to promptly treat all infectious pathologies and diseases of the reproductive system.
- You can't get too cold.
- It is important to eat rationally and maintain a sleep-wake schedule.
- You should stop smoking and drinking alcohol.
- At the first suspicion of “problems” in the functioning of the reproductive system, you should immediately consult a doctor.
Hypofunction of the uterine appendages significantly affects not only reproductive health, but also health in general. At the first signs of the development of the disease, it is recommended to immediately consult a doctor.
Causes and forms of primary hypofunction
Primary ovarian hypofunction includes:
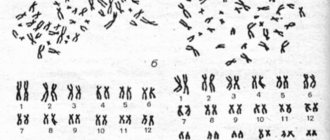
- Structural changes in the gonads caused by disturbances at the stage of their formation or intrauterine development. The reason for this may be infectious diseases and exogenous intoxications suffered by the mother in the first trimester of pregnancy. A genetically determined pathology is also possible; in such cases they speak of gonadal dysgenesis. The most common chromosomal abnormalities in this case are mosaicism and testicular feminization syndrome with the formation of a female phenotype in the presence of a male karyotype and male gonads.
- Exhausted ovarian syndrome, also called premature menopause. Pathogenetically, it is manifested by too rapid “consumption” of the intrauterine ovarian reserve, which becomes the reason for a woman’s abnormally early transition to menopause.
- Resistant ovarian syndrome.
- Postnatal damage to the gonads, accompanied by a significant decrease in their size or the development of massive sclerosis (scarring). This also includes the consequences of operations on the appendages, including the condition after resection or removal of the ovary.
Often, all forms of postnatal damage are included in the syndrome of exhausted ovaries, considering them as the etiological forms of this disease. And prenatal dysgenesis can also generally be considered a condition that contributes to the development of early menopause. However, pathogenetically these are still different forms of primary ovarian hypofunction. Therefore, it is advisable to separate them.
Secondary hypofunction: what it is and why it occurs
Secondary ovarian hypofunction is a decrease in their activity as a result of any endocrine disorders in a woman while the gonads are intact. In this case, any factors that negatively affect the functioning of the hypothalamic-pituitary system can be important. After all, it is she who has a hormone-mediated effect on the “switching on” of the gonads at puberty and their subsequent work during the reproductive period.
The main forms of secondary ovarian hypofunction are:
- Isolated hypogonadotropic dysfunction leading to the development of hypogonadotropic hypogonadism. This pathology can be congenital (including hereditary) or develop after puberty.
- Functional disorders in the hypothalamic-pituitary-ovarian system, not associated with structural damage to any link. They can be caused by stress, neurotic disorders, hyperprolactinemia, increased testosterone levels of any origin. Often, the cause of secondary ovarian hypofunction is non-physiological weight loss - due to extreme diets, anorexia nervosa, severe somatic diseases and intoxications.
- Organic lesions of the hypothalamic-pituitary system. Possible causes of this pathology include various intracranial tumors, previous meningoencephalitis, neuroinfections and traumatic brain injuries, consequences of radiation, a number of poisonings, ischemic changes in the hypothalamic region.
Most often, functional forms of secondary ovarian hypofunction are diagnosed. Most of them are potentially reversible, subject to adequate correction of the primary pathological condition that caused an imbalance in the functioning of the hypothalamic-pituitary-ovarian system.
Reasons for appearance
Naturally, ovarian hypofunction does not appear on its own. It is provoked by certain reasons. Pathology can be primary or secondary. In the first case, it is congenital, provoked by a violation of intrauterine development due to the transmission of infectious or inflammatory diseases by the mother. As for secondary hypofunction, the disorder occurs under the influence of such factors:
- psychoemotional disorders;
- serious infections;
- a sharp decrease in estrogen levels, as well as disruption of the functionality of the endocrine system;
- metabolic problems;
- genetic predisposition;
- infectious or inflammatory pathologies of the reproductive system (salpingoophoritis);
- chronic fatigue syndrome or body exhaustion;
- chemotherapy treatment;
- structural changes in the tissues of the appendages;
- poor nutrition, following excessively strict diets, sudden weight loss;
- uncontrolled use of hormonal contraceptives;
- diseases of the hypothalamic-pituitary zone due to mental or mechanical trauma.
A disorder can also develop as a result of chronic adnexitis. The causes of ovarian hypofunction must be clarified, otherwise treatment of the pathological condition will be useless.
Clinical picture
Symptoms of ovarian insufficiency can appear in a woman at any age during the reproductive period. And in some forms - already when the girl enters puberty, while she will not actually experience the “ripening” of the reproductive system and its transition to the functionally active stage.
The main symptoms of ovarian hypofunction:
- Menstrual irregularities. They may include oligomenorrhea, primary or secondary amenorrhea. The severity of the disorders depends on the degree of endocrine imbalance and hormonal deficiency. In congenital forms, there is a delay in the onset of menarche and puberty.
- Pathology of pregnancy, if a woman’s conception nevertheless occurred naturally or with the use of assisted reproductive technologies. In the absence of drug correction of ovarian hypofunction, a woman has a high risk of spontaneous abortion in the early stages and miscarriage. Therefore, in the first trimester they are often diagnosed with a threatened miscarriage with partial detachment of a normally implanted ovum.
- Anovulation and related reproductive disorders such as infertility. Depending on the form and age of onset of ovarian insufficiency, problems with natural conception may be primary or secondary. In this case, the endocrine type of infertility is most often diagnosed.
- Subatrophy of the mucous membrane of the vagina and vulva, reduction in the thickness of the functional layer of the endometrium. With long-term and severe ovarian insufficiency, involution of the initially normally developed internal genital organs is also noted. If existing disorders lead to a lack of adequate endocrine stimulation of the ovaries during puberty, sexual infantilism is diagnosed. In this case, there is hypoplasia (underdevelopment) of the uterus, vaginal walls, appendages, external genitalia with weak expression of secondary sexual characteristics.
- Psychovegetative disorders arising due to severe estrogen deficiency, similar to the symptoms of menopause. They are not a mandatory sign; they are characteristic mainly of exhausted ovarian syndrome. With other forms of ovarian hypofunction, such striking characteristic vegetative manifestations usually do not occur.
Hypoestrogenia in ovarian insufficiency can also contribute to the development of osteoporosis with a tendency to atraumatic fractures, pathological weight gain, deterioration of skin and hair, prolonged or recurrent affective disorders of the depressive spectrum, and atherogenic imbalance of blood lipids.
Of course, such conditions are usually not dominant in the clinical picture. Often their appearance is not associated with the woman’s existing ovarian hypofunction. And symptomatic treatment carried out in such cases will not give the expected effect without correcting the underlying pathology.
The most common pathologies
Unfortunately, almost all ovarian diseases that are not cured in time or cannot be treated lead to infertility. Asymptomatic diseases are especially dangerous when nothing bothers a woman. Therefore, women are recommended to visit a gynecologist at least once a year.
Ovarian pathologies are common in women, regardless of their age.
Inflammation
It develops when an infection enters and develops in the ovaries, often accompanied by the development of inflammation of the fallopian tubes and ligaments. Infection can be caused by viruses, microbes and protozoa.
The inflammatory process in the ovaries is accompanied by a number of symptoms:
- Pain in the lower abdomen on the left or right.
- Increase in body temperature to 38-39 degrees.
- Malaise, headache, nausea.
- Vaginal discharge with an unpleasant odor, its yellowish color indicates the presence of pus.
Inflammation of the ovaries is highly treatable; after tests and examination, the doctor prescribes antibiotics of a certain group (penicillins, cephalosporins and others), and after some time the disease recedes. During treatment (on average up to a month), abstinence from sexual intercourse is recommended.
Ovarian cancer
The most unpredictable disease, as it has almost no symptoms at an early stage. Ovarian cancer can metastasize to other nearby organs, so the disease is dangerous and unpredictable. Treatment is prescribed by an oncologist after ultrasound, tomography, and tests to confirm the diagnosis.
Next, surgery is performed to remove the tumor or organ. Most often, if ovarian cancer is not detected in a timely manner, all organs of the woman’s reproductive system are removed.
Cyst
The disease is very common, has many different types, and is most often removed surgically or carefully monitored by a doctor until it worsens.
The disease is often asymptomatic and is detected by ultrasound. Complications of the disease include twisting of the cyst stalk and rupture of its wall. They are already accompanied by severe pain in the lower abdomen, high fever and nausea.
They require immediate help (surgery). In most cases, cysts can disappear on their own, and some can degenerate into malignant formations.
Benign tumors
They can develop in any part of the ovary, are characterized by painful attacks in the lower abdomen (dull pain), or do not bother at all. If detected, surgery is prescribed to remove the tumor.
Diagnostics
Diagnosis of ovarian insufficiency syndrome should be aimed not only at confirming the fact of a decrease in the functional activity of the ovaries. The most important task of the examination is to clarify the level of damage, which will allow to differentiate primary and secondary forms of this pathology. If possible, a search for the cause of existing disorders is also carried out. It is also necessary to identify the degree of secondary changes in target organs that develop against the background of hypoestrogenism.
The examination should include:
- General somatic and gynecological examination. This will allow you to assess the severity of secondary sexual characteristics and the condition of the mucous membrane of the vulvovaginal area, approximately determine the size of the uterus, and identify symptoms of masculinization in the patient.
- Assessment of basic endocrine profile. To do this, determine the level of LH, FSH, progesterone, estrogen, prolactin. If menstrual function is preserved, an analysis for each hormone is carried out at the appropriate phase of the cycle. For amenorrhea - several times over a certain period of time to dynamically assess fluctuations.
- Carrying out pharmacological tests to help determine the level of disorders (ovarian or hypothalamic-pituitary) and the sensitivity of ovarian tissue to hormones. Tests are used with the administration of estrogen, progesterone, clomiphene, and human chorionic gonadotropin.
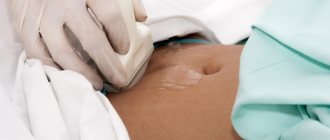
- Ultrasound of the pelvic organs to determine the size of the internal genital organs, the thickness of the functional layer of the endometrium, the condition and structure of the ovaries.
- X-ray of the skull with a targeted examination of the area of the sella turcica, which is important in cases of secondary ovarian hypofunction.
- MRI of the pituitary gland (also if hypothalamic-pituitary disorders are detected, if radiography did not provide enough information).
A comprehensive examination is the basis for competently drawing up a subsequent treatment plan and assessing the possibility of restoring fertility.
The mechanism of regulation of ovarian function in normal conditions and in cases of hypothalamic-pituitary dysfunction.
A symptom of infertility is a manifestation of the depletion of the compensatory capabilities of certain parts of the reproductive regulation system. In 50 - 70% of cases, infertility is determined by the condition of the wife, in 20 - 25% of cases - by the condition of the husband. In 10 - 30% of cases, mixed forms occur, and in 2 - 5% of cases the cause of infertility is not clear (2). In the structure of female infertility, endocrine disorders occur in 35 - 40% of cases, dysfunction of the fallopian tubes - in 30 - 40% of cases, uterine factors - in 10%, cervical - in 7 - 10% of cases, vaginal - in 6%, extragenital - in 1%, mental - in 1% of cases. The same or similar data are given in most gynecological manuals (10).
The basis of a woman’s reproductive system is the hypothalamus-pituitary-ovary axis, the proper functioning of which ensures the maturation of a full-fledged egg, adequate preparation of the endometrium for pregnancy, tubal transport of gametes, fertilization, implantation and maintenance of early pregnancy.
The highest organ of regulation of the hypothalamic-pituitary-ovarian axis is the central nervous system, which, through a whole complex of direct and feedback connections, ensures the stability of the reproductive system when the internal and external environment changes (14, 21). To date, more than 36 peptides have been discovered that regulate GnRH secretion (28). Based on the fact that all the main neuroendocrine circles are directly or indirectly connected with the immune system and, in addition to the endocrine centers, connect areas of the brain with lymphoid tissue, some researchers are currently talking not about the neuroendocrine, but about the neuro-immune-endocrine system for regulating reproduction (30, 33).
The releasing factor for the two major gonadotropins, LH and FSH, is Gn-RH, a decapeptide synthesized by Schally and Guillemin in 1977. GnRH is synthesized in the arcuate nucleus of the mediobasal hypothalamus and enters the portal blood flow system of the pituitary gland in a pulsed manner. To ensure normal secretion of gonadotropins, it is sufficient to maintain a stable frequency of release of physiological amounts of Gn-RH. Changing the frequency of Gn-RH release changes not only the amount of LH and FSH secreted by the pituitary gland, but also their ratio, while even a tenfold increase in the concentration of Gn-RH leads only to a slight decrease in FSH release and does not change LH secretion in any way.
The frequency of Gn-RH release in humans is 1 release every 70 - 90 minutes and corresponds to a number of biorhythms (alternation of sleep phases, fluctuations in glomerular filtration rate and gastric secretion, frequency of hot flashes during menopause, etc., which confirms Kleitmann's hypothesis about the existence of general rhythm with a periodicity of about 90 minutes, which is related to the basal rest-activity cycle (20), which is explained by geophysical reasons (22, 37).The main factors regulating the frequency of GnRH release are opiates and alpha-blockers (6, 12, 13).The pulse rhythm generator - the arcuate nucleus - does not need any influence from other parts of the nervous system to maintain its normal operation (1).Under physiological conditions, the pulse generator receives information about the release of gonadotropins by the pituitary gland through a short feedback system, since special sphincters regulate pressure gradients in the portal blood flow system, and part of the blood from the pituitary gland does not flow into the cavernous sinus, but back into the hypothalamus, which ensures a very high local concentration of pituitary hormones in the hypothalamus (31). The synthesis and secretion of LH and FSH in the pituitary gland are carried out by the same cells (7). On the surface of gonadotropes there are receptors for Gn-RH, the density of which depends on the level of steroid hormones in the blood and on the concentration of Gn-RH. The connection of Gn-RH with the receptor causes a massive influx of calcium ions into the cell, which after a few minutes leads to the release of LH and FSH reserves into the bloodstream. In addition, GnRH stimulates the synthesis of LH and FSH and maintains the integrity of gonadotropes (40). Changes in pulse generator frequency alter the ratio of LH and FSH secreted by the pituitary gland (24). Thus, an increase in rhythm leads to a significant increase in the release of FSH and a decrease in the release of LH. Frequency modulation of information ensures speed and reliability of regulation of the reproductive system and its resistance to interference (4, 36). In the luteal phase, progesterone, through endogenous opiates, reduces the frequency of the pulse generator, and this action is determined not by the concentration of progesterone, but by the duration of its effect. Estradiol, acting on the hypothalamus and gonadotropes (increasing the density of Gn-RH receptors), increases the amplitude of the LH / FSH wave (16, 39).
Progesterone stimulates the formation of an inhibitor in the hypothalamus, which eliminates this effect of estradiol (29, 35). This eliminates the possibility of an LH peak during the luteal phase, which could disrupt the maturation of a cohort of follicles for the next menstrual cycle (11).
Gonadotropins are the main regulators of the synthesis and secretion of sex steroids. The site of production of sex steroids in the body can be the follicular complex (theca interna, theca externa, granulosa and oocyte), the corpus luteum and the ovarian stroma. The usefulness of cyclic changes that ensure the preparation of a woman’s body for pregnancy is determined by the quality of selection and maturation of the dominant follicle. The main patterns of folliculogenesis were established by the working group of Professor Hodgen at the turn of the 1970s and 1980s (11). They proposed the terms recruitment, cohort, selection, establishment of dominance. Recruitment is the name given to the process of transition of follicles from the primordial stage to the antral stage, since only from this time the maturation process becomes dependent on the action of gonadotropins. The recruitment process is determined by intraovarian factors and occurs constantly, but only those follicles that are recruited in the last 4 days of the luteal phase of the previous cycle will be able to form a cohort - a group of follicles from which the dominant one will emerge (39). The number of recruited follicles is most likely determined by the level of gonadotropins in the late luteal phase and the local concentration of progesterone in the ovary, which explains the alternation of ovulation in the right and left ovaries. The growth of the cohort of follicles in the early follicular phase is explained by favorable conditions for the ratio of LH and FSH and local concentrations of estrogens and androgens. The action of LH and FSH on the follicle is strictly specialized: LH stimulates the process of de novo androgen synthesis by theca cells and has virtually no effect on granulosa cells, and FSH activates the aromatase system of the granulosa, which converts androgens synthesized in the theca into estradiol (15).
Estrogens and FSH inhibit atresia of the preantral follicle and stimulate the proliferation of granulosa cells, the synthesis of FSH receptors and the induction of LH receptors, starting at the periphery of the follicle and moving towards the center. The appearance of LH receptors in granulosa cells of large follicles is a prerequisite for the synthesis of progesterone by the corpus luteum. LH, through stimulation of androgen synthesis, limits and reduces the synthesis of receptors for FSH, LH, estradiol in follicle cells. The synergistic action of LH and FSH in the early follicular phase causes a significant increase in estrogen secretion by the ovary. This in turn induces an increase in the LH/FSH index, which shifts the synthesis of sex steroids in the follicles towards the preferential formation of androgens. In the normal course of events, by the 8th day of the menstrual cycle, the selection of the dominant follicle ends, the main property of which is the ability to enhance estrogen production in conditions of FSH deficiency and completely suppress the development of other follicles of the cohort with the help of intraovarian and hypothalamic-pituitary connections (8, 11, 18, 42) . If for any reason the dominant follicle dies, recruitment must occur again, since no other follicle in this cohort can take on the role of the dominant one. An important role in the process of suppression of other follicles is played by the polypeptide regulator inhibin, which selectively suppresses the secretion of FSH, and the follicle-regulating protein, which selectively suppresses the aromatase activity of granulosa.
On days 12–14 of the cycle, the dominant follicle is responsible for almost all the production of estradiol in large quantities, which causes a peak in LH and FSH, which is the cause of ovulation.
The FSH peak in the middle of the cycle is important for the normal functioning of the corpus luteum, ensuring the induction of the synthesis of LH receptors in the granulosa cells of the preovulatory follicle.
In healthy women, proper development of the dominant follicle causes:
- adequate production of estradiol, ensuring the maturation of the endometrium and the accumulation of progesterone receptors in its epithelium and the maturation of cervical mucus;
- full ovulation;
- preparation of receptors for LH in granulosa, which should turn into the corpus luteum.
Thus, the quality of the luteal phase is determined primarily by the processes occurring in the first phase of the cycle. According to the 1976 WHO classification, all disorders of the endocrine function of the ovaries are divided into 7 large groups:
- hypogonadotropic normoprolactinemic insufficiency;
- normogonadotropic normoprolactinemic insufficiency;
- hypergonadotropic insufficiency;
- anatomical form of amenorrhea;
- hyperprolactinemia;
- hyperprolactinemia;
- volumetric processes in the hypothalamic-pituitary region that do not change the secretion of prolactin (5).
The vast majority of patients with impaired ovarian function who seek treatment for infertility belong to the 2nd group of disorders according to the WHO classification - eugonadotropic hypothalamic-pituitary dysfunction. Clinically, in this group one can distinguish subgroup 2a - patients with spontaneous menstrual cycles - and subgroup 2b - patients with amenorrhea. Patients of subgroup 2a are characterized by insufficiency of the luteal phase due to impaired maturation of the dominant follicle, impaired ovulation and impaired function of the corpus luteum, as well as anovulatory menstrual cycles, characterized by the fact that the dominant follicle matures, but does not ovulate; during the period of atresia of the dominant follicle, luteinization of the granulosa and theca occurs , accompanied by sharply reduced progesterone production. In this case, the basal temperature either does not increase or increases slightly.
Luteal phase deficiency, anovulation and amenorrhea are usually an expression of the degree of endocrine disruption and often act as stages of one process (34).
The typical expression of type 2 ovarian failure is an increase in the LH/FSH ratio, accompanied by a small (compared to hormone-producing tumors) adrenal and/or ovarian hypersecretion of androgens (5). Traditionally, such forms of hypothalamic-pituitary-ovarian dysfunction were classified as polycystic ovary syndrome. However, this term is disputed by a number of authors. On the one hand, ovarian enlargement can occur with Cushing's syndrome, andrenogenital syndrome, with hormone-producing tumors, and sometimes in healthy adolescents. On the other hand, women with typical manifestations of this syndrome may have normal sized ovaries. In addition, with this syndrome, anatomical changes in the ovaries are only a consequence of disrupted hormonal interactions in the body.
Therefore, it is proposed to call this type of pathology hyperandrogenism syndrome with chronic anovulation (19). In terms of hormonal changes, the most characteristic signs are a LH/FSH ratio greater than 2 and an increase in the level of androgens (testosterone, androstenedione and DHEA-S) in the peripheral blood (17).
Compared with healthy women, in whom the main estrogen in the circulating blood is estradiol, women with hyperandrogenism syndrome have significantly increased estrone levels, which may exceed the concentration of estradiol. The main source of increased estrone levels in such patients is peripheral aromatization of androstenedione. Constant and monotonous production of estrone sensitizes the pituitary gland to the action of Gn-RH, resulting in an increase in the LH/FSH ratio secreted by the pituitary gland. In turn, high LH levels lead to excessive stimulation of the ovarian stroma and theca, resulting in excessive androgen production. Under these conditions, both the process of selection of the dominant follicle and its usefulness are sharply disrupted, which leads to opsomenorrhea, anovulation, luteal phase deficiency and amenorrhea (9, 27).
Hypothalamic-pituitary dysfunction in type II ovarian failure is a purely functional disorder in which positive feedback is disrupted. The etiology of hyperandrogenism syndrome with chronic anovulation is still unknown. It has been proven that heredity, central catecholamine disturbances, mental stress and obesity play an important role in the development of the syndrome (32).
Dysfunction of the adrenal cortex plays an important role in the development of the disease. In a significant proportion of patients, the adrenal glands are very sensitive to ACTH stimulation. In this regard, it has been hypothesized that the pituitary gland secretes a specific hormone that stimulates androgens of the adrenal cortex with a molecular weight of about 60,000 (32). Some patients are heterozygous carriers of the C-21-hydroxylase defect (38).
In addition, increased production of androgens by theca cells can also be caused by increased insulin levels due to the overlap of insulin specificity and local growth factors (3). Consequently, hirsutism and hyperandrogenism may be a manifestation of profound metabolic disorders.
Hyperandrogenic ovarian insufficiency is characterized by an increase in the amplitude and frequency of pituitary LH bursts (41).
An important role in pathogenesis is played by the influence of androgens on the level of testosterone and estrogen binding protein (TESB). With hyperandrogenism and obesity, the synthesis of TESH in the liver decreases, which leads to an increase in the active concentrations of estrogen and testosterone in the blood, as a result of which the manifestations of hyperandrogenism intensify. There are indications that non-hereditary intrauterine influences play an important role in the development of the syndrome, and that maternal hyperandrogenism may have an adverse effect on the maturation of various fetal enzyme systems (25). With hyperandrogenism syndrome, the ratio of norepinephrine and dopamine changes, and the resulting dopamine deficiency leads to increased LH release.
Impaired development of the dominant follicle and ovulation in normogonadotropic ovarian failure leads to the development of NLF (23).
There are 5 reasons for the development of NLF: impaired follicle maturation; insufficient stimulation of LH in the 2nd phase of the cycle; insufficient and/or delayed luteinization of the preovulatory follicle; mild forms of hyperprolactinemia; hyperandrogenism of various origins (23). The hormonal manifestation of NLF is a decrease in the production of progesterone by the corpus luteum, accompanied by normal or increased secretion of estradiol (relative hyperestrogenism). At the cellular level, NLF is manifested by increased cell divisions (endrometry, mammary gland, myometrium). Clinically, NLF manifests itself as premenstrual syndrome, menstrual irregularities, decreased fertility, benign breast tumors and uterine fibroids. The causes of infertility in NLF are insufficient endometrial maturity, which impedes normal implantation, and insufficient progesterone levels to support early pregnancy (26).
- The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). -Plant TM, Krey LS, Moossy J., McCormack JT, Hess DL, Knobil E. //Endocrinology, 1978, v. 102, N 1, p. 52-62.
- Baltzer J., Mickan H. Kern Gynäkologie. 4. Aufl. Stuttgart: Thieme, 1985. -685 S.
- Barbieri RL, Ryan KJ Hyperandrogenism, insulin resistance and acanthosis nigrans syndrome: A common endocrinopathy with distinct pathophysiological features. //American Journal of Obstetrics and Gynecology, 1983, v. 147, N 1, p. 90-101.
- Bohumil RJ Pulsatile variations in hormone levels. //Biorythms and human reproduction. — Ferin M., Halberg F., Richart RM, Van de Wiele RL (Eds) New York: Wiley, 1974, p. 107-131.
- Breckwoldt M. Störungen der Ovarialfunktion. //Reproductionsmedizin. -Bettendorf J., Breckwoldt M. (Hrsg.). Stuttgart; New York: Fisher, 1989, pp. 258-266.
- Central electrophysiological correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. -Wilson RS, Kesner JS, Kaufman JM, Uemura T., Akema T., Knobil E. //Neuroendocrinology, 1984, v. 39, N 3, p. 256-260.
- Childs GV Functional ultrastructure of gonadotropes: a review. //Morphology of hypothalamus and its connections. -Ganten D., Pfaff D. (Eds.). Berlin: Springer, 1986, p. 49-98.
- Correlation of human follicular fluid inhibin activity with spontaneous an
- Davis OK, Ravnikar V. Induction of ovulation with Clomiphen Citrate. //Reproductive endocrine therapeutics. – Barbiery L., Schiff I. (Eds.). New York: AR Liss, Inc., 1988, p. 1-24.
- Diedrich K., Wildt L. Neue Wege in der Behandlung ovarieller Funktionsstorungen. Teil 1. //Neue Wege in der Diagnostik und Therapie der Weiblichen Sterilität. -Diedrich K., Hrsg. -Stuttgart: F. Enke, 1987, p. 26-40.
- DiZerega GG, Hodgen GD Folliculogenesis in the primate ovarian cycle. //Endocrine review 1981, v. 2, N 1, p. 27-49.
- The effect of morphine on the electrophysiological activity of the hypothalamic luteinizing hormone-releasing hormone pulse generator in the rhesus monkey. -Kesner JS, Kaufman G., Wilson RC, Kuroda G., Knobil E. //Neuroendocrinology, 1986, v. 43, N 6, p. 486-488.
- Electrophysiological manifestation of luteinizing hormone releasing hormone pulse generator activity in the rhesus monkey: influence of a adrenergic and dopaminergic blocking agents. -Kaufman JM, Kesner JS, Wilson RS, Knobil E. //Endocrinology, 1985, v. 116, N 4, p. 1327-1333.
- Everett JW Central neural control of reproductive functions of the adenohypophysis. //Physiology review, 1964, v. 44, p. 373-431.
- Falck B. Site of production of estrogens in rat ovary as studied by microtransplants. //Acta physiologica Scandinavica, 1959, v. 163, N 1, p. 1.
- Ferin M., van Vugt D., Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. //Recent progress in hormone research, 1984, v. 40, p. 441-485.
- Givens JR, Andersen RN, Umstot ES Clinical findings and hormonal responses in patients with polycystic ovarian disease with normal versus elevated LH levels. //Obstetrics and gynecology, 1976, v. 47, N 4, p. 388-394.
- Hoffmann F. Untersuchunden über die hormonale Regulation der Follikelreifung im Zyklus der Frau. //Geburtshilfe und Frauerheilkunde, 1961, Bd. 21, S. 554-560.
- Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr.; V. Davaian, 2nd edition. - Oradell: Medical Economics Books, 1986. -IX, 688 p.
- Kleitmann N. Sleep and wakefulness. - Chicago: Chicago University Press, 1963. -250 p.
- Lakoski JM Cellular electrophysiologycal approaches to the central regulation of female reproductive aging. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DKRassin (Eds.). -New York: Liss, 1989, p. 209-220.
- Lavie P., Kripke DF Ultradian circa 1½ hour rhythms: A multioscillatory system. //Life sciences, 1981, v. 29, N 24, p. 2445-2450.
- Lobo RA Polycystic ovary syndrome. //Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr. and V. Davajan, 2nd edition. — Oradell: Medical Economics Books, 1986, p. 319-336.
- Leyendecker G., Wildt L., Plotz EJ Die hypothamische Ovarialinsuffizienz.//Gynäkologe, 1981, Bd. 14, N 2, S. 84-103.
- Lobo RA Polycystic ovary syndrome. //Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr. and V. Davajan, 2nd edition. — Oradell: Medical Economics Books, 1986, p. 319-336.
- Mauvais-Jarvis P., Kutten F. Insuffisance gonadotrope dissociée (anovulation et dysovulation)
- The microinvironment of the human antral follicle: Interrelationships among the steroid levels in human antral fluid, the population of granulosa cells and the status of the oocyte in vivo and in vitro. -McNatty KP, Smith DM, Makris A., Osathanonolh R., Ryan KJ //Journal of clinical endocrinology and metabolism, 1979, v. 49, N 6, p. 851-860.
- Miller BT Peptide modulation of luteinizing hormone releasing hormone secretion. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DK Rassin (Eds.). New York: ARLiss, Inc., 1989, p. 255-271.
- Mode of action of progesterone in the blockade of gonadotropin surges in the rhesus monkey. -Pohl CR, Richardson WD, Marshall G., Knobil E. //Endocrinology, 1982, v. 110, N 4, p. 1454-1455.
- The Neuro-immune-endocrine connection. - Cotman C., Brinton RE, Galaburda A., McEwen BC -New York: Raven Press, 1986. -150 p.
- Page RB Pituitary blood flow. //American journal of physiology, 1982, v. 243, N 6, p. 427-442.
- Parker LN, Odell WB Control of adrenal androgen secretion. //Endocrine review, 1980, v. 1, N 4, p. 392-410.
- Perez-Polo JR Introduction: Neuroimmune modulation of reproductive function. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DK Rassin (Eds.). -New York: AR Liss, 1989, p. 307-309.
- Plotz EJ Differentialdiagnose und Therapie ovarieller Funktionsstörungen: Richtlinien fur die Praxis. //Gynäkologe, 1981, Bd. 14, N 2, S. 145-148.
- The pulsatile pattern of gonadotropin secretion and follicular development during the menstrual cycle and in women with hypothalamic and hyperandrogenic amenorrhea. -Wildt L., Schwilden H., Werner G., Roll C., Brensing KA, Vuckhaus J., Böhr M., Leyendecker G. //Brain and pituitary peptides II. — G. Leyendecker, H. Stock, L. Wildt (Eds.). -Basel: Karger, 1983, p. 28-36
- Rushton WAH Peripheral coding in the nervous system. //Sensory communication. -W.A. Rosenblith (Ed.). -New York: Wiley, 1961, p. 20-30.
- Shapiro S. Compass on the 90-minutes sleep-dream cycle. //Sleep and dreaming. -Hartman E. (Ed.) -Boston: Little and Brown, 1970, p. 40-49.
- An update of congenital adrenal hyperplasia. — New MI, Dupont B., Pang S., Pollack M., Levine SL // Recent progress in hormone research, 1981, v. 37, p. 105-181.
- Wildt L. Die endokrine Kontrolle der Ovarialfunktion und die Pathologie endokriner Ovarialfunktionsstörungen. // Neue Wege in Diagnostik und Therapie der weiblichen Sterilität. -Hrsg. von K. Diedrich. - Stuttgart: Enke, 1987, S. 1-25.
- Wildt L. Hypothalamus. //Reproductionsmedizin. —Hrsg. von Bettendorf G., Breckwoldt M. - Stuttgart: Fischer, 1989, S. 6-22.
- Yen SSC The polycystic ovary syndrome. //Clinical endocrinology, 1980, v. 12, N 2, p. 177-207.
- Zeleznik AJ, Schuler HM, Reichert LC, Jr. Gonadotropin binding sites in the rhesus monkey ovary: Role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. //Endocrinology, 1981, v. 109, N 2, p. 356-362.
d induced follicular maturation. -Murrs RP, Lobo JD, Campeau JD, Nakamura RM, Brown J., Ujita EL, DiZerega GS //Journal of Clinical Endocrinology and Metabolism, 1987, v. 64, N 1, p. 148-152.
. //Medecine de la reproduction. Gynécologie endocrinienne. -Paris: Flammarion, 1982, p. 305-319.
.
Tags: menstrual cycle
Fertility in ovarian failure
The ovaries are the most important element of the female reproductive system, responsible for the maturation of eggs (oocytes). They are the basis for reproduction of the species. All other processes in the reproductive organs are aimed at creating favorable conditions for conception and subsequent pregnancy. And this is ensured by the cyclically changing level of sex hormones, which are in mutual balance and are also produced in the ovaries. Therefore, a pronounced decrease in the functional activity of normally developed female gonads or significant damage to their tissue always leads to a decrease in a woman’s fertility.
Infertility is associated both with a violation of the monthly process of maturation and ovulation of oocytes, and with insufficient “preparedness” of the uterus for the implantation of fertilized eggs. So problems with the onset and maintenance of pregnancy are an obligatory component of ovarian insufficiency syndrome.
Whether it is possible to become pregnant with ovarian hypofunction depends on the severity and potential reversibility of the changes a woman has. Moreover, in most cases, successful conception requires not a course of treatment, but continuous maintenance treatment. And sometimes the patient requires not only drug correction, but also the use of high-tech assisted reproductive techniques. If ovarian hypofunction leads to primary amenorrhea with severe hypoplasia of the genital organs, the prognosis for achieving fertility is usually questionable. In this case, even if it is possible to achieve egg maturation, the services of a surrogate mother are often required.
Non-hormonal therapy
Naturally, it is impossible to do without hormonal treatment, however, it is necessary to use other methods of therapy:
- Physiotherapeutic procedures. They help stimulate the development of the genital organs. You should use exactly those procedures that help improve blood circulation.
- Therapeutic physical education.
- Vitamin therapy. This method of treatment is necessary during the active formation of cyclic changes in the ovaries. The patient will have to take vitamins C, E, folic acid, magnesium and zinc.
- Traditional methods. There are various gynecological preparations that are used to activate the formation and maturation of follicles. Also, folk remedies reduce the severity of symptoms. A popular collection is one that contains boron uterus. It has long been considered a “female” herb. Rhodiola rosea, leuzea, red brush, rosehip, mistletoe, licorice, calamus help well. The “Scarlet Flower” collection is quite popular.
Hypofunction is a complex multifaceted problem. Any patient can suffer, regardless of age. Timely treatment of pathology can eliminate most of its forms.
How to treat ovarian hypofunction
Treatment of ovarian hypofunction can be aimed at solving several problems:
- Normalization of the menstrual cycle.
- Restoring fertility (if a woman is planning a pregnancy). This can be achieved by correcting the hormonal background (bringing it closer to the physiological level), activating the maturation and ovulation of eggs, normalizing the size of the uterus and the thickness of the functional layer of the endometrium.
- Initiation of the onset of puberty with the appearance of secondary sexual characteristics. This is relevant for congenital hypofunction of the ovaries of a primary or secondary nature, including those caused by chromosomal abnormalities.
- Correction of manifestations of estrogen deficiency: subatrophy and dryness of the vulvovaginal mucosa, vegetative-vascular and psycho-emotional disorders, osteoporotic bone changes.
Treatment for ovarian hypofunction should be prescribed taking into account the type of disorder and the objectives. At the same time, in some cases, in young patients, it may be advisable to carry out step-by-step therapy, when dyshormonal disorders are first corrected and only then the issue of the possibility of pregnancy is decided.
In case of primary ovarian failure, the basis of treatment is estrogen replacement therapy, which, if necessary, is supplemented with measures to correct the consequences of hypoestrogenism. Initially, estrogenization is carried out using monocomponent hormonal drugs, and subsequently they switch to combined estrogen-gestagen options. After achieving a clinical effect, it is recommended to transfer the patient to low-dose drugs to minimize the side effects of such long-term maintenance therapy. For this purpose, for example, Yarina is appointed. This treatment is carried out until the woman reaches the average age of menopause.
Low-dose estrogen-gestagen contraceptive drug "Yarina"
In the secondary form of ovarian hypofunction, drugs are selected taking into account sensitivity to gestagens and estrogens, which is determined through tests. In girls with delayed puberty, an stimulant regimen is initially used to initiate growth of the reproductive organs and trigger menarche. Subsequently, they are given maintenance therapy that allows them to imitate the natural female hormonal levels.
Planning pregnancy is possible only after the menstrual cycle has been restored and cyclic changes in ovarian tissue have been achieved. In most cases, this is achieved by stimulating the maturation of the egg and subsequent provocation of its ovulation (for more details, see the article at the link). The therapeutic regimens used are selected individually, often as part of an IVF protocol.
Non-hormonal treatment
Different hormone therapy regimens are the basis for correcting any form of ovarian insufficiency. But other means can additionally be used in treatment. These include:
- Physiotherapy. Its use is relevant at the stage of stimulation of the development of the genital organs. Preference is given to techniques aimed at improving blood circulation in the pelvic organs.
- Exercise therapy, the task of which is also to improve the blood supply to the internal genital organs.
- Vitamin therapy, most often prescribed at the stage of active correction of the ovaries, provoking cyclic changes and maturation of oocytes in them. In this case, special cyclic schemes for the use of certain minerals and vitamins are used: zinc, magnesium, vitamins C, E, group B, folic acid.
- Use of medicinal plants and their preparations. Treatment of ovarian hypofunction with folk remedies can be aimed at reducing the severity of psychovegetative manifestations of hypoestrogenism and activating folliculogenesis. Various mixtures are used based on boron uterus, Rhodiola rosea, red brush, Leuzea, mistletoe, rose hips, marsh calamus, licorice and other plants.
Ovarian hypofunction is a fairly frequently diagnosed and multifactorial pathology. Moreover, patients of any age can be susceptible to it: both those already having children and those just entering reproductive age. Many forms of ovarian insufficiency are amenable to drug correction, and women, with adequately selected therapy, often even manage to conceive and safely carry a child. But do not forget that treatment should only be selected by a doctor after a comprehensive, full examination and clarification of the pathogenetic type and degree of ovarian hypofunction.
Treatment
Therapy sets itself certain goals.
First of all, this is the normalization of a disrupted menstrual cycle and the restoration of reproductive capabilities if a woman still plans to become a mother.
Hormone therapy is used here. Depending on the situation, doctors stimulate ovulation. This tactic is relevant if the problem is congenital. Another factor is the restoration of estrogen balance.
Methods are adopted based on the general clinical picture and the results of the examination. For young patients, it is advisable to carry out therapy in several stages. Initially, correction of dyshormonal disorders is carried out, and only then it is decided whether it is possible to get pregnant.
If the primary type of disease is identified, then it is appropriate to restore the estrogen balance. Certain medications are used for this type of treatment. To achieve maximum effect, the dosage should be low, as there is a risk of side effects. The contraceptive drug Yarina is often prescribed.
In the case of diagnosing a secondary form, drugs should be selected based on individual sensitivity to estrogens and gestagens. These indicators are determined by taking samples. For those girls who do not have their first menstruation for a long time, a scheme is used to stimulate the growth of the reproductive organs.