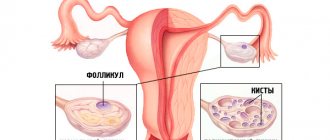
What it is?
Resistant ovarian syndrome is considered a poorly understood pathology. Its main feature is amenorrhea and infertility, which occur with normally formed secondary sexual characteristics, high levels of gonadotropins and macro- and microscopically unchanged monads. It is worth noting that 1969 was a fatal year, because then resistant ovarian syndrome was first studied and described. But even in our time this topic is not yet sufficiently covered.
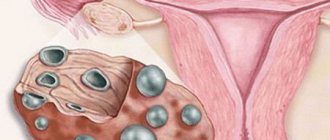
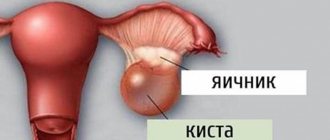
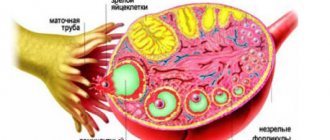
This syndrome accounts for 1.9-10% of cases among various forms of amenorrhea. Resistant ovarian syndrome is a follicular, specific type of ovarian failure that is different from follicular amenorrhea. Resistant ovaries are characterized by the fact that each follicle moves very slowly. As a result of such sedentary activity, anovulation occurs, which subsequently provokes infertility. Resistant ovarian syndrome is also called Savage syndrome and the syndrome of “dumb”, resting, paralyzed, insensitive or refractory ovaries.
Causes of resistant ovarian syndrome
To date, the causes of the development of resistant ovarian syndrome have not been sufficiently studied. There is a high probability that its development is due to genetic defects in the receptor apparatus of the follicles.
A large amount of data is also provided that indicates that this pathology is autoimmune in nature. Resistant ovarian syndrome results in the appearance of antibodies in the serum that block the sensitivity of FSH receptors in the ovaries. As practice shows, this syndrome often occurs in parallel with diseases such as Hashimoto's thyroiditis, diabetes, hemolytic anemia, myasthenia gravis, hypoparathyroiditis, alopecia, platelet purpura, etc.
Iatrogenic factors have their influence on the development of resistant ovarian syndrome: the use of cytostatics and immunosuppressants, ovarian resection, and radiotherapy. With tuberculosis, actinomycosis, sarcoidosis, mumps, ovarian tissue is affected, which can also trigger the development of a disease such as resistant ovarian syndrome.
Symptoms of resistant ovarian syndrome
Disruption of the menstrual cycle, and then cessation of menstruation, is a characteristic sign of resistant ovarian syndrome in women who are not more than 35 years old. Quite often, as practice shows, this pathology is hereditary in nature for disorders of menstrual and reproductive function. In addition, the syndrome can occur after suffering a severe viral infection, taking large doses of sulfonamides, exposure to stress, etc.
Quite often, resistant ovarian syndrome is accompanied by oligomenorrhea, which can last from 3 to 10 years. Another characteristic sign of pathology is the absence of vegetative-vascular disorders (sweating, palpitations, hot flashes, etc.), which are typical for exhausted ovarian syndrome and menopause.
Typically, a patient diagnosed with resistant ovaries has the correct female body type and well-developed secondary sexual characteristics. Every woman should be careful, because this pathology provokes the development of fibrocystic mastopathy.
Diagnosis of pathology
A study of the medical history and examination by a gynecologist can suggest the presence of resistant ovarian syndrome. A final diagnosis can be made after the following manipulations:
- Laboratory research. With ROS, a decrease in progesterone and estrogens and an increase in FSH and LH are detected.
- Ultrasound of the pelvic area, which determines the following:
- the reproductive organ is of normal size,
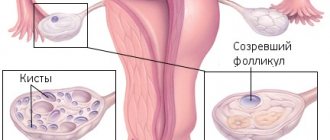
- the dominant follicle is absent, all formations are immature,
- the ovaries retain typical sizes or are slightly smaller than normal; inside them there are many follicles with a diameter of no more than 4-6 mm.
- Hormonal tests that analyze the body's response to medication.
- Laparoscopy and biopsy. As a result of the first study, multiple translucent follicles with a diameter of no more than 6 mm are determined, and the second study identifies immature follicles.
Consultation with a gynecologist for menstrual irregularities is mandatory. Determining the concentration of hormones in the blood is an endocrinological diagnostic method that allows us to identify the causes of menstrual irregularities, however, the diagnosis of “resistant ovarian syndrome” is made if the patient does not have any other pathology, manifested by amenorrhea and the inability to conceive a child.
Diagnosis of resistant ovarian syndrome
It should be understood that the diagnosis of resistant ovarian syndrome must necessarily include laboratory and instrumental studies. Only through an integrated approach will it be possible to distinguish this syndrome from other diseases with similar symptoms.
A gynecological examination can reveal signs of hypoestrogenism - hyperemia and thinning of the mucous membranes of the vagina and vulva. Also, the “pupil” symptom is observed.
A pelvic ultrasound can determine the size of the uterus, endometrium and ovaries. Ultrasound examination is excellent for examining follicles up to 5-6 mm.
Diagnosis of resistant ovarian syndrome is impossible without a hormone study, because this way you can see some kind of disorder. Normally, blood plasma contains low levels of prolactin and estradiol. This disease is also characterized by an increase in the level of prostaglandin E2 by 3-4 times, testosterone by 3-10 times, and cortisol by 2 times.
The most information about resistant ovarian syndrome is provided by hormonal tests. A gestagen test is carried out, the first result of which is almost always positive, and the next one is negative. This pathology is characterized by a positive result in a cyclic mode. This fact indicates that feedback remains between the ovary and the hypothalamic-pituitary region.
Diagnostic laparoscopy is ideal for seeing visible follicles in the ovaries. In turn, biopsy and histological studies can reveal the presence of preantral and primordial follicles in the biopsy specimen.
Diagnostics
Diagnostics is based on:
- registration of clinical and laboratory manifestations of hyperandrogenism;
- assessment of menstrual and ovulatory function;
- morphology of the ovaries by ultrasonography.
2.1 Physical examination
Assessment of hirsutism using the Ferriman-Gallwey Scale (F-G scale).
In classic PCOS, the prevalence of hirsutism is 75%; in the Caucasian and Negroid races, ≥8 points on the F-G scale are pathognomonic; in representatives of Southeast Asia, ≥3.
Routine assessment of acne and alopecia is not recommended; they can be criteria for PCOS only in combination with ovulatory dysfunction or polycystic disease.
A clinical marker of insulin resistance (IR) in patients with PCOS is acanthosis nigroid, manifested by papillary pigmentary degeneration of skin areas.
Measuring height and weight with calculating BMI and determining the type of obesity based on waist circumference (WC).
2.2 Laboratory diagnostics
total and free testosterone in blood serum , the latter is the most informative.
Not recommended:
- ELISA determination of total testosterone;
- direct method - free testosterone.
For the diagnosis of hyperandrogenemia and subsequent monitoring, it is advisable to calculate the free testosterone fraction from the testosterone level in CCHS.
Screening for IGT and type 2 diabetes using a 2-hour oral OGTT with 75 g of glucose.
glycosylated hemoglobin is determined to screen for carbohydrate metabolism disorders in PCOS .
OGTT is performed every 3-5 years or more often for central obesity, significant weight gain and/or symptoms of diabetes.
2.3 Instrumental diagnostics
To diagnose polycystic ovaries (PCOS) with ultrasonography , it is necessary to detect more than 12 follicles with a diameter of 2-9 mm in at least one ovary and/or a more reliable criterion - an ovarian volume of more than 10 ml.
Transvaginal ultrasound is performed:
- with regular menstruation in the early follicular phase;
- for oligo/amenorrhea at any time or 3-5 days after progesterone-induced menstruation.
If the dominant follicle is more than 10 mm or the corpus luteum, the ultrasound is repeated in the next cycle.
For cysts or ovarian asymmetry, additional research is required.
These criteria should not be applied to women receiving COCs.
With transvaginal ultrasound with a high-frequency sensor (≥8 MHz), the criteria for PCOS are the presence of 25 or more follicles from 2 to 10 mm in the ovary and/or an ovarian volume of more than 10 cm3.
2.4 Diagnosis of anovulation
Diagnostic criteria for ovulatory dysfunction:
1. NMC with a cycle of less than 21 or more than 35 days;
2. with a preserved menstrual cycle on days 20-24, serum progesterone is below 3-4 ng/ml.
Chronic anovulatory dysfunction is the absence of ovulation in 2 out of three cycles.
2.5 Differential diagnosis
It is necessary to exclude:
- diseases of the thyroid gland (with hypothyroidism, TSH is higher than normal and free thyroxine , hyperthyroidism - TSH is below 0.1 mU/l);
- hyperprolactinemia;
- non-classical form of congenital adrenal cortex dysfunction (CAD) for 17-OH-progesterone more than 400 ng/dl or 13 nmol/l, with a “borderline” value of more than 200 ng/dl or 6 nmol/l - ACTH test ;
- insulin resistance according to the euglycemic hyperinsulinemic clamp test or glucose tolerance test ( EGTT ), or the HOMA and QUICKI indices ;
2.7 Diagnosis of metabolic syndrome
Criteria for metabolic syndrome (MS) in women with PCOS:
1. increase in WC in Caucasians is more than 88 cm, in Asians it is more than 80 cm; 2. triglycerides (TG) ≥ 1.7 mmol/l (≥ 150 mg/dl); 3. HDL <1.3 mmol/l (4. SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or previously diagnosed hypertension; 5. fasting plasma glucose ≥ 5.6 mmol/l (≥100 mg/dl).
To confirm MS, 3 out of 5 criteria must be present.
2.8 Obstructive sleep apnea syndrome (OSA)
It is recommended to identify patients with PCOS and overweight or obesity.
If OSAS is suspected, a polysomnographic study is performed, followed by referral to a specialized health care facility.
2.9 Assessing the risk of cardiovascular disease (CVD) in women with PCOS
At each visit - measurement of blood pressure, WC and registration of BMI.
If the lipid profile is normal, repeat the test every 2 years or more often if you gain weight.
Risk group for PCOS with the presence of at least one of the following factors:
- obesity;
- smoking;
- hypertension;
- dyslipidemia;
- subclinical atherosclerosis;
- impaired glucose tolerance;
- family history.
High-risk group for PCOS:
- MS;
- Type 2 diabetes;
- clinical atherosclerosis;
- kidney pathology;
- OSA.
2.10 Fatty hepatosis and non-alcoholic steatohepatitis
Routine diagnosis of fatty liver disease and non-alcoholic steatohepatitis in patients with PCOS is not recommended.
2.11 Depression
Screening for anxiety and depressive disorders is recommended for all patients with PCOS .
Treatment of resistant ovarian syndrome
Treatment of resistant ovarian syndrome is a complex and time-consuming process, since its etiopathogenesis in most cases remains unclear.
The first thing prescribed for this pathology is two- or three-phase HRT, which is designed to eliminate estrogen deficiency, normalize the menstrual cycle and reduce the level of gonadotropic hormones. A young patient who is on contraception should take medications such as Trisequence, Clymene, Femoston, Klimonorm, Premella Cycle, etc. If a woman is over 50 years old, then in her case HRT is continuous (Climodien, Cliogest, Livial).
In case of resistant ovarian syndrome, non-drug methods include abdominal and intravaginal ultraphonophoresis and spa acupuncture, which acts exclusively on the receptor areas of the ovaries.
In cases where normal ovulatory function is not restored, the IVF method with a donor egg is used. Women over 40 years old are recommended to undergo annual mammography, densitometry to exclude osteoporosis, pelvic ultrasound, as well as studies of lipoproteins and blood cholesterol. Treatment should be carried out under the supervision of a gynecologist and other doctors. It is also important to follow all their recommendations and indications.
Treatment
Treatment goals:
- elimination of manifestations of androgen-dependent dermatopathy;
- normalization of body weight and correction of metabolic disorders;
- restoration of the ovulatory menstrual cycle and fertility;
- prevention of late complications of PCOS.
3.1 Conservative treatment
Monotherapy with combined hormonal contraceptives (CHC):
- COOK;
- patch;
- ring is first-line therapy for NMC, hirsutism and acne.
For women with PCOS who are not interested in pregnancy, any method of contraception is recommended.
The use of CHCs does not have a negative impact on future fertility in most people; the benefit-risk ratio must be considered.
In case of contraindications or intolerance to CHCs, 2nd line therapy in patients with PCOS and irregular menstruation is metformin . The instructions for the drug do not contain this indication.
(Treatment of hirsutism in PCOS)
Low-dose CHCs are recommended .
For moderate hirsutism, CHC monotherapy .
If CGC monotherapy is ineffective or severe hirsutism, use a combination of CGC with antiandrogens .
Evaluation of treatment effectiveness after 6 months.
Monotherapy with antiandrogens is recommended only in cases of contraindications or intolerance to COCs.
When prescribing antiandrogens, reliable contraception is necessary.
In cyclic or continuous mode use:
- 50-100 mg per day of spironolactone (this indication is not included in the instructions);
- 10-100 mg per day of cyproterone acetate ;
- finasteride for women is not registered in the Russian Federation;
- flutamide is hepatotoxic and therefore not recommended.
In addition to drug therapy for hirsutism, there are cosmetic methods of hair removal, optimally photoepilation .
The use of metformin is not recommended.
(Acne treatment for PCOS)
Along with CGCs, systemic antibiotics of the tetracycline group, macrolides and isotretinoin are used, prescribed by a dermatologist.
Isotretinoin has a pronounced teratogenic effect, so reliable contraception is necessary.
(Treatment of obesity in PCOS)
Therapeutic lifestyle modification (TLM) with exercise and diet is recommended
Weight loss against the background of TMJ helps normalize menstruation and metabolic parameters without having a significant effect on hirsutism.
Metformin is not recommended.
The use of metformin is possible in PCOS with type 2 diabetes or ineffectiveness of TMZ in IGT.
Pharmacotherapy for obesity is indicated for BMI ≥30 kg/m2 or BMI ≥27 kg/m2 in the presence of one of the complications:
- hypertension;
- dyslipidemia;
- Type 2 diabetes;
- OSA.
Bariatric surgery is indicated:
- BMI ≥40 kg/m2;
- ≥35 kg/m2 with complications of obesity.
Behavioral therapy to reduce food intake and increase physical activity is essential for treatment.
The effectiveness of a hypocaloric diet against the background of sibutramine increases and the level of androgens decreases more significantly.
Moderate weight loss has also been reported with orlistat .
In the pharmacotherapy of obesity, the benefit/risk ratio is unfavorable; the feasibility of routine use is questionable.
(Treatment of anovulation in PCOS)
With PCOS there is a high risk of anovulatory infertility.
Ovulation induction should be preceded by TMJ and treatment of obesity.
1st line therapy for anovulatory infertility - clomiphene citrate (CC) 50-100 mg/day for 5 days, starting from 2-5 days of the menstrual cycle. The maximum daily dose is 150 mg.
CC treatment for 6 ovulatory cycles, stimulation efficiency is 70-80%, conception rate is 22% per cycle, cumulative live birth rate is 50-60%.
Predictors of CC ineffectiveness:
- increased free testosterone index and BMI;
- amenorrhea;
- increased ovarian volume.
Against the background of CC, additional administration of hCG in the middle of the cycle does not increase the likelihood of pregnancy.
Ovarian hyperstimulation syndrome (OHSS) develops in 10% of patients using CC.
Routine use of metformin for ovulation induction is not recommended and its effectiveness has not been proven.
In PCOS with infertility, metformin is used only for carbohydrate metabolism disorders or for the prevention of ovarian hyperstimulation syndrome during assisted reproductive technologies (ART).
If clomiphene citrate is ineffective or intolerant, the second line is to stimulate ovulation with gonadotropins or laparoscopy .
It is preferable to gradually increase the dose of gonadotropin (Step-up mode), the starting dose of FSH is 37.5-50 IU/day; if there is adequate follicular growth, it does not change; if there is no growth after a week, it increases by 50%.
More clinical experience is required to use initial doses of FSH 100-150 IU/day (Step-down regimen).
The duration of use of gonadotropins is no more than 6 cycles with monitoring of the ovarian response.
For the induction of ovulation, a combination of gonadotropin-releasing hormone agonists and gonadotropins is not recommended.
3.2 Surgical treatment
Indications for laparoscopy in PCOS with infertility:
- resistance to clomiphene citrate;
- high LH levels;
- inability to monitor when using gonadotropins;
- combination with conditions requiring laparoscopic surgery (endometriosis, tubo-peritoneal infertility).
Laparoscopic drilling is not used to correct irregular menstrual cycles or hyperandrogenism.
The effectiveness of laparoscopic drilling and gonadotropins is comparable.
of monopolar electrocautery and laser is comparable .
To achieve the effect, 4 ovarian punctures are enough; a larger number increases premature ovarian failure.
If 12 weeks after laparoscopy there is no ovulation, CC induction is required. After 6 months of clomiphene citrate, gonadotropins can be used.
Prevention of resistant ovarian syndrome
Today, gynecology, due to insufficient knowledge and complexity of the development of resistant ovarian syndrome, cannot identify specific ways to prevent this disease. The only thing that is recommended is to exclude adverse iatrogenic effects: radiation, infections, drug intoxication.
In any case, if irregularities occur in the menstrual cycle, you should consult a doctor and undergo a full gynecological examination. Timely detection of resistant ovarian syndrome will prevent its development and minimize the negative impact on a woman’s reproductive health. Remember this, otherwise your health condition may deteriorate sharply.
Consequences and prognosis
For women of reproductive age, the main complication of resistant ovarian syndrome is infertility. In addition to the inability to get pregnant on your own, other consequences arise:
- psychological problems;
- deterioration in the quality of intimate life;
- osteoporosis;
- premature aging of the body.
By choosing the right treatment tactics, most complications can be avoided. Problems arise only with the restoration of reproductive function.
Resistant ovarian syndrome often occurs in women with a family history. But the exact reasons for its occurrence are unknown, so it is almost impossible to prevent its development. When the syndrome appears, it is important to adhere to the prescribed treatment regimen. This will avoid premature aging of the body and prevent the development of complications.