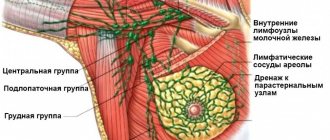
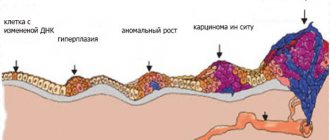
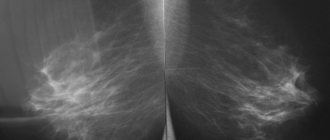
Due to high morbidity and mortality, difficulties in diagnosing and treating tumor processes are one of the main problems in medicine, and their correct and timely diagnosis, carried out in accordance with modern classification systems, allows one to avoid a number of serious complications. It has been shown that the prevalence of malignant neoplasms of the thyroid gland from 2006 to 2021 increased from 66 to 105 per 100,000 population, and by the end of 2021, the number of patients registered for this pathology was 154,824 [1].
According to the new International Classification of Tumors of the Endocrine System, for encapsulated thyroid tumors with the absence of capsular and/or vascular invasion, as well as changes in the nuclei of tumor cells characteristic of papillary thyroid cancer, the classification category “non-invasive follicular thyroid tumor with papillary-type nuclei” has been introduced. (NIFOYAPT). This category is included in the group “other encapsulated thyroid tumors of follicular structure” [2]. Studies have shown a slow and favorable clinical course of NIPHO, therefore at this time it is classified as a benign neoplasm of the thyroid gland. This indicates that treatment of patients with NIFOT can be reduced to the minimum, namely hemithyroidectomy, and total thyroidectomy and radioiodine therapy do not have any advantages for this pathology. In addition to the lack of psychological burden of a cancer diagnosis, the number of complications that can arise after total thyroidectomy is reduced, as is the risk of secondary tumors after radioiodine therapy [3]. This approach increases the responsibility of the attending physician and pathologist when diagnosing NIFOP and requires the presence of a set of clear morphological criteria. Below is a clinical observation, which we consider useful for diagnosing and determining treatment tactics for this pathology.
A 36-year-old woman was diagnosed with a nodular formation in the left lobe of the thyroid gland. Upon palpation of the left lobe, a node measuring 2x2x1 cm with clear contours, soft-elastic consistency, and movable when swallowing was determined. The lymph nodes of the neck are not enlarged. Based on the results of functional tests and using radioimmunoassay, the following abnormalities were identified: increased level of thyroid-stimulating hormone - 6.6 μIU/ml (with the norm being 0.27-4.0 μIU/ml), decreased level of free T4 - 0.71 ng/dl (with a norm of 0.8-2.1 ng/dl), an increased level of antibodies to thyroid peroxidase - 393.6 IU/ml (with a norm of 0-35 IU/ml). According to ultrasound examination, in the left lobe of the gland, closer to the upper pole, a heterogeneous, isoechoic formation, surrounded by a capsule, measuring 22x13x15 mm with pronounced vascularization, and a region of hypoechoic structure along the periphery was discovered (Fig. 1).
Rice. 1. Ultrasound examination of the thyroid gland. Regional lymph nodes are not enlarged. The patient underwent a fine-needle aspiration biopsy of the nodular formation, based on the results of which the conclusion was made: the material is multicellular, a large number of follicular structures were found, in some of which colloid was detected. In some fields of view, there are single atypical microfollicular structures of polymorphic thyrocytes with layering of nuclei, the presence of large, polymorphic, predominantly centrally located nucleoli (Fig. 2).
Rice. 2. Microfollicular structures of the tumor, atypical microfollicular structure, Pappenheim staining, ×200. Rice. 2. Microfollicular structures of the tumor, atypical microfollicular structure, Pappenheim staining, ×200. A left-side hemithyroidectomy was performed and macroscopic examination in the area of the upper pole of the gland revealed an encapsulated nodular formation measuring 22×13×15 mm, gray-yellow in color. Thyroid tissue was fixed for 24 hours in a 10% formaldehyde solution, after which tissue fragments 2-3 mm thick were placed in cassettes. Histological sections 4-5 µm thick were stained with hematoxylin and eosin. Microscopic examination revealed that against the background of chronic thyroiditis, a tumor was identified in the left lobe of the gland, having a microfollicular and normofollicular structure, surrounded by a focally thickened connective tissue capsule due to fibrosis, intact throughout its entire length (Fig. 3).
Rice. 3. Intact tumor capsule. Rice. 3. Intact tumor capsule. Despite the follicular structure, two components can be distinguished in the tumor: the first occupies mainly the central parts of the tumor, makes up about 40% and is represented by follicles lined with monomorphic thyrocytes with hyperchromic nuclei, the second occupies the peripheral parts of the tumor, makes up about 60%, and is represented by follicles lined polymorphic thyrocytes with cleared nuclei, in which pseudo-inclusions, furrows and layering of cell nuclei are determined (Fig. 4).
Rice. 4. Intranuclear inclusion in a tumor cell. Rice. 4. Intranuclear inclusion in a tumor cell. The tumor follicles of both components are filled with eosinophilic colloid, and the tumor stroma is represented by thin layers of connective tissue with mild lymphoid infiltration. No foci of tumor invasion into blood and lymphatic vessels, psammoma bodies or mitotic figures were found in tumor cells. Based on pathohistological examination, a diagnosis of NIPHO was made. An immunohistochemical study of malignancy markers in tumor cells revealed focal expression of galectin-3 (Fig. 5),
Rice. 5. Focal expression of galectin-3 in tumor cells, ×400. Rice. 5. Focal expression of galectin-3 in tumor cells, ×400. HBME-1 (Fig. 6)
Rice.
6. Focal expression of HBME-1 in tumor cells, ×400. Rice. 6. Focal expression of HBME-1 in tumor cells, ×400. in the tumor component, consisting of cells with papillary-type nuclei, which indicates the immunophenotypic heterogeneity of the tumor in a morphologically homogeneous cellular composition. A molecular genetic study of DNA isolated from paraffin blocks was carried out using allele-specific real-time polymerase chain reaction (PCR) to identify the V600E mutation of the BRAF
, as well as mutation-specific PCR followed by Sanger sequencing to characterize the mutational status of the 15th exon of the
BRAF
, 2-4 exons of
the NRAS
and
KRAS
and 2-3 exons of the
HRAS
. No mutations were identified in any of the genes studied. Also, no RET/PTC1 and RET/PTC3 translocations were detected.
Non-invasive breast cancer
Intraductal carcinoma (ductal carcinoma in situ)
It is essentially intraductal carcinoma and is considered a precursor to invasive carcinoma. This does not mean that every invasive breast cancer (BC)
goes through the stage of cancer in situ, but the risk of developing invasive forms against the background of cancer in situ is high.
Surgery and/or radiation therapy can cure patients. This is the form of cancer that it is desirable to actively detect during mammography examinations.
Improvements in screening in many European countries have resulted in an annual increase in in situ cancer of 3.9% from 1973 to 1983 and 17.5% from 1983 to 1992. The rate of this disease in 1973 was 2.4 per 100 thousand female population, and in 1992 - 15.8.
Cancer in situ often presents as separate lesions separated by layers of breast tissue. Depending on nuclear atypia, cell polymorphism, the number of atypical mitoses, the presence of foci of necrosis in the ducts, calcifications and their number, cancer in situ is divided into three degrees of differentiation. Therefore, the pathological report should contain a description of the tumor according to the following parameters.
Pathological description of intraductal carcinoma:
• tissue architectonics (number and size of microfoci of carcinoma and the background against which development occurred); • severity of nuclear atypia; • presence of necrosis; • nodular or diffuse lesions (size and number of nodes); • distance of carcinoma foci from the resection margin; • presence of microcalcifications (their location directly in the carcinoma focus or outside).
Intraductal carcinoma of high differentiation or low grade of malignancy (low grade ductal carcinoma in situ G1)
Carcinoma in situ Gl consists of small monomorphic cells growing in papillary, cribriform, solid structures within the mammary ducts.
The nuclei are polymorphic, some are hyperchromic with clear nucleoli, mitotic figures are rare. Proliferations may be observed in one or more ducts. Between them are layers of mammary gland tissue or, more often, ductal neoplasia of varying degrees of differentiation. The lesions are usually small, up to a maximum of 2 mm in diameter.
Microcalcifications, the presence of desquamated cells inside the ducts, necrosis and comedo-type structures are not typical for this stage of differentiation. Micropapillary and cribriform structures are most commonly noted (Figure 25).
Photo 25. Cancer in situ G1. Tumor cells form cribriform intraductal structures. Hematoxylin-eosin, x 200
Intermediate grade ductal carcinoma in situ G2
Carcinoma in situ G2 consists of small polymorphic cells growing inside the mammary ducts in the form of papillary, cribriform, solid structures. Unlike the previous option, cancer in situ G2 is more characterized by solid structures, comedo-type structures, as in acne cancer (photo 26).
Photo 26. Cancer in situ G2. In the area of cancer, desquamation of the epithelium and the formation of a “comedo” type structure are noted. In the tissue surrounding intraductal cancer there are changes characteristic of lobular ductal hyperplasia of the mammary gland. Hematoxylin-eosin, x 200
The nuclei are polymorphic, hyperchromatic with coarsely dispersed chromatin, and mitotic figures are more often noted. Foci of necrosis and microcalcifications are possible, but these signs are not necessary. The carcinoma lesions are small, as in G1 carcinoma in situ.
Intraductal carcinoma of low differentiation (high grade ductal carcinoma in situ G3)
Cancer in situ G3, unlike previous differentiation options, has larger lesions, often up to 5 mm, but in addition to large lesions, isolated small lesions are possible, sometimes less than 1 mm.
The structure of the arrangement of cells in the duct is the same, that is, the presence of papillary, cribriform and solid growth forms with a predominance of solid structures. In G3 carcinoma in situ, the cells are more polymorphic, with many polygonal cells of different sizes noted. Large cells predominate, especially in areas of proliferation. The nuclei are hyperchromatic, atypical, with many mitotic figures. Necrosis and microcalcifications are often noted, which can be located focally or diffusely in the lumen of the ducts. The cytological characteristics of in situ G3 cancer cells are close to those of invasive carcinoma cells (photo 27-28a).
Photo 27. Cancer in situ G3. In the field of view there are several ducts with cancer in situ, in one of them there are microcalcifications, in the upper duct there is a focus of necrosis. Hematoxylin-eosin, x 100
Photo 28. Cancer in situ G3. Tumor cells are large, fill the lumen of the ducts completely, forming solid structures. Hematoxylin-eosin, x 200
Photo 28a. Ductal carcinoma in situ. The myoepithelial layer is preserved as a continuous layer (brown line in the photo). Expression of smooth muscle actin (clone HHF35, produced by DAKO). Immunohistochemical staining, EnVision imaging system, DAB chromogen. Myoepithelial cells in the form of a continuous line along the basement membrane, some of these cells entered the papillary structure inside the duct, x 200
The main thing for verifying cancer in situ of any degree of differentiation is the reliable determination of the absence of tumor microinvasion into the stroma. To do this, it is necessary to examine a large number of sections and use the immunohistochemical method to stain the basement membrane and myoepithelial cells.
It is necessary to pay attention to changes in the structure of the stroma, which responds to tumor invasion and lymphoplasmacytic infiltration around the foci of tumor invasion.
It should be noted that the morphological code in the International Classification of Oncological Diseases for cancer in situ of all degrees of differentiation is the same - 8500/2.
Specific variants of in situ carcinoma are versiform cell, apocrine, signet ring, neuroendocrine, squamous and clear cell. These variants are reflected in the WHO classification (2003), but are detected very rarely.
Proliferative activity is now often tested using the Ki 67 antibody. In in situ G3 carcinomas with comedo structures, the proliferation index is 13%, in in situ G1 carcinomas an average of 4.5%, and in high-grade micropapillary carcinomas it can often be null.
For the differential diagnosis of ductal and lobular neoplasia, immunohistochemical studies are used. E-cadherin is always expressed in ductal neoplasias, and cytokeratin 1/5/10/14 (clon CK34BetaE12) is negative in most cases (92%). In lobular neoplasia the opposite is true. There is no expression of E-cadherin, but cytokeratin 1/5/10/14 (clon CK34BetaE12) is almost always detected.
Clinical picture
With mammary cancer, the symptoms are specific, depending on the type of tumor, and common to any type of cancer process. Specific signs:
- Invasive ductal carcinoma - the nipple is deformed, pathological fluid oozes from it.
- Lobular cancer - a painful compaction with a bumpy surface occurs. The breast shrinks and the nipple retracts.
- Inflammatory carcinoma – has signs of mastitis, which can make diagnosis difficult.
- Paget's carcinoma - chronic eczema occurs in the nipple area.
Common symptoms and signs of the disease include pyrexia, loss of appetite and weight, and anemia. The mammary glands become asymmetrical and may have different sizes and shapes. Symptoms also appear on the skin, it changes color and becomes reddish, yellow, with a blue tint. It is worth remembering that in the initial stages of development, carcinoma can occur secretly, without any pain or visual signs, so the pathology is often diagnosed in an advanced form.
Intracystic papillary carcinoma
It is detected more often in older women (on average 65 years), accounting for 2% of all cases of breast cancer.
The size of the tumor can be large - from 0.4 to 10 cm, on average 2 cm. First of all, the tumor is represented by a cyst and papillary cancer in the form of a small area in the cyst or replacing almost the entire cavity of the cyst. The morphological structure is characteristic of intrapapillary cancer, that is, it is represented by fibrovascular rays, devoid of myoepithelial lining and covered with neoplastic epithelium, corresponding to G1 differentiation.
Atypical epithelium forms solid, cribriform, papillary structures. The myoepithelial lining is preserved only in the wall of the non-tumor cyst. Mucin production is possible (photo 29).
Photo 29. An area of intracystic papillary cancer with pronounced signs of secretion. Hematoxylin-eosin, x 100
There are areas of metaplasia, areas of cells of neuroendocrine differentiation. A characteristic feature is the absence of tumor invasion into the surrounding stroma (photo 30).
Photo 30. Section of the cyst wall of intracystic papillary cancer. The epithelial lining of the cyst is malignant and is represented by micropapillary structures. There are no signs of tumor invasion into the fibrous capsule of the cyst. Hematoxylin-eosin, x 100
The morphological code in the International Classification of Oncological Diseases for papillary cancer inside a cyst is 8504/2.
Prognosis and prevention
The prognosis for breast cancer depends on the stage at which therapy was started.
- With stage 1 carcinoma, five-year survival is ensured for ninety women out of a hundred,
- If stage 2 breast cancer is detected, the five-year survival rate drops to sixty-six.
- At the third stage, the survival rate is no more than forty-one percent,
- After stage 4 treatment, only ten percent of women can expect a five-year survival rate.
For malignancy only up to grade G2, the prognosis is relatively favorable. Prevention of recurrence from cancer infiltrating the second breast is only possible by removing both breasts. Prevention is about avoiding risk factors.
Invasive cervical cancer – what is it? Cervical cancer ranks third among all malignant neoplasms that affect women of reproductive age. At the initial stage of the pathological process, non-invasive cervical cancer is determined. In this case, the atypical cells grow slowly and spread throughout the cervical mucosa. Invasive cervical cancer is characterized by intense cell growth. It can spread to other organs and impair their functioning. The process of transition from non-invasive cervical cancer to invasive lasts up to 20 years, but it is inevitable. Do gynecologists at the Yusupov Hospital conduct timely diagnosis of the disease using the latest equipment from the world's leading manufacturers?
Early treatment of non-invasive and microinvasive cervical cancer can improve the prognosis of five-year survival. The Yusupov Hospital employs a team of highly qualified specialists: oncologists-gynecologists, chemotherapists, radiologists. Doctors treat non-invasive, pre-invasive and invasive cervical cancer according to ASCO and NCCN standards. Professional care is provided by nurses who know the specifics of the cancer process and are attentive to the wishes of patients and their relatives.