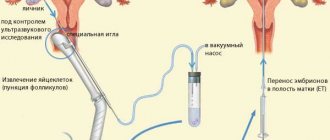
Many modern women postpone having a child due to education and career advancement, confident that they will always be able to turn to methods of assisted reproductive technology as a last resort.
At the same time, most of them do not realize what processes take place every month in their ovaries and, when deciding to become mothers at an older age, they encounter significant problems.
Would you like to make an appointment?
How can one assess the condition of the ovaries, the so-called “ovarian reserve”, which indicates the number of follicles located in the ovarian tissue?
Causes and symptoms of decreased ovarian reserve
A decrease in ovarian reserve is a reduction in the number of eggs and a deterioration in their quality. A natural decrease in follicular reserve occurs after 35-38 years, but there are other reasons for this phenomenon.
The reasons for the decrease in ovarian reserve are:
- age over 35 years,
- history of gynecological surgeries,
- malignant neoplasms,
- genetic tendency to early menopause,
- drug or alcohol addiction,
- smoking.
Signs of decreased ovarian reserve before menopause:
- irregularity of menstruation,
- bloody vaginal discharge between cycles,
- a feeling that throws you into heat, then into cold,
- problems with conceiving within a year with regular sexual activity without using condoms,
- increased fatigue.
Low ovarian reserve be increased . Over the course of life, it is gradually “wasted.” The most “suitable” eggs for conception mature before the age of 30-35, but it is believed that a woman’s fertility begins to decline after 30 years. Normally, menopause occurs around age 50, but the ability to become pregnant is lost earlier.
A small number of eggs potentially ready for fertilization significantly reduces the likelihood of conception. The chances of becoming pregnant using IVF, with a decrease in the ovarian follicular reserve, are slightly higher than naturally. The Teramoto protocol is noticeably effective. If there is a significant shortage of eggs, it is recommended to use the services of a donor.
Why is the number of eggs decreasing?
The quality and quantity of eggs in the follicular reserve is extremely important. The reason for the decrease in ovarian reserve is a natural physiological process. The optimal childbearing age is 20-30 years, that is, after 30 years, the likelihood of getting pregnant is reduced by 2 times. Pathological conditions, inflammatory reproductive diseases, unhealthy lifestyle, adverse effects on the ovaries and other processes cause a decrease in reserve.
Predisposition at the genetic level
Genetic predisposition implies some kind of disorder in the female line. For example, various menstrual disorders, infertility, early menopause, or problems with gestation and pregnancy that were observed in the mother, grandmother or sisters.
Intrauterine decrease in the initial follicular reserve
A decrease in the initial reserve is caused by severe pregnancy:
- gestosis conditions,
- intrauterine fetal hypoxia,
- infection.
Chronic intoxication
Intoxication of the body is a factor in reducing ovarian reserve. This is explained by the fact that regular contact with toxic substances is the result of the appearance of chronic intoxication. Industrial waste, agricultural chemical solutions, occupational hazards and household toxic components are causes of egg damage.
Premature ovarian failure syndrome
Premature ovarian failure syndrome, a condition that is manifested by amenorrhea for more than 4 months, under the age of 40, high levels of pituitary gonadotropins, especially FGS (follicle-stimulating hormone) and a decrease in estrogen in the blood. This syndrome adversely affects the ovarian tissue, resulting in its depletion.
Chemotherapy and radiation therapy
Radiation rays negatively affect the function of a woman’s reproductive system. There are factors that contribute to damage to ovarian function:
- age of the woman receiving therapy
- dose of radiation (4 Gray rarely causes changes in the functioning of a woman’s ovaries, and a dose of 5 to 10.5 Gray over the age of 40 contributes to the appearance of persistent menopause),
- localization of irradiation.
Chemotherapy, in some cases, causes irreversible menopause. Influencing factors:
- patient's age,
- drug dose,
- the selected drug (antimetabolite cytarabine, melphalan, cyclophosphamide, vinblastine and others).
Inflammatory diseases
If inflammatory diseases of the reproductive system (acute endometritis, adnexitis, oophoritis, salpingoophoritis, endometriosis, adenomyosis) are not treated in a timely manner, the inflammation becomes chronic, leading to changes in blood supply, immunological disorders and ultimately cell death.
Surgical damage
Surgical interventions on the pelvic organs have a strong impact on the reduction of ovarian reserve.
Women very often have a history of undergoing surgery for infertility, as well as other female reproductive diseases. An operation such as ovarian resection significantly reduces the reserve. If the volume of resection is significant, cell hypoxia develops due to impaired blood supply. A critical decrease in eggs and follicles occurs during adnexectomy.
Tobacco smoking
Smoking causes significant harm to the body. Toxic substances in tobacco smoke negatively affect the life of eggs. Often, the reduction in follicular reserve is observed in women who smoke about 3 times more than in non-smoking women. Also, smokers shorten the period of menopause by 2 years.
Products containing chemical compounds
The high content of chemically toxic substances in food, medicines, poor ecology and even in water negatively affects the human body.
Endometriosis
Endometriosis of the third and fourth stages is the leading cause of infertility. But not only the growth of tissue on the ovaries leads to damage to the reproductive function, but also the amount of resection performed during the treatment of the ovaries for endometrioid cysts.
Polycystic ovary syndrome
As with endometriosis, polycystic ovary syndrome results from surgery, thereby reducing ovarian reserve. With this syndrome, there is a change in the assessment of ovarian reserve, a specific morphological picture, as well as the development of anovulation.
Ovarian reserve assessment
Ovarian reserve assessment should be performed in women over 35 years of age. As the body ages, not only does the number of eggs decrease, but their quality also deteriorates. Among other things, the likelihood of successful embryo implantation is reduced. For men over 48 years of age who wish to have children, medical genetic counseling is recommended.
Indications for assessing ovarian reserve:
- chemotherapy in the past
- infertility of unknown nature,
- if you plan to use reproductive technologies,
- with menorrhagia in premenopause, when a decision on therapy is made.
The goal of any IVF is to select the best genetic material and successfully transfer the embryo. To do this, it is necessary to select material with a reserve (9-13 follicles, and then 7-11 oocytes and 5-9 embryos). To predict the success of IVF, it is necessary to determine the quality of the response to ovulation stimulation.
Comprehensive assessment of ovarian reserve. Click to enlarge
Hormone tests to assess ovarian reserve
Many women dream of increasing their fertility. The number of eggs available to the patient’s reproductive system can be calculated in various ways. The following describes hormonal studies, the results of which can be used to judge the quality of the ovarian reserve:
- FSH in the follicular phase. During menopause, the level of this hormone exceeds 30 mIU/l. A good ovarian reserve is indicated by an indicator from 3 to 8 mIU/l. A slight excess of the FSH norm with a constant menstrual cycle often indicates a decrease in follicular reserve and occurs 5-6 years before menopause. After 35 years, a hormone concentration of more than 10 mIU/l in the first phase of the cycle most often guarantees an insufficient response to stimulation. Significant fluctuations in FSH indicate a reduction in follicular reserve. An insufficient response to stimulation is often observed with a small amount of luteinizing hormone on the third day of the cycle (hereinafter referred to as DC).
- Estradiol. If it is more than 250 pg/ml, then this indicates a lack of ovarian reserve (even when FSH is within normal limits). Patients aged 38-42 years whose estradiol level on the third DC (day of the cycle) is less than 80 pg/ml can count on a successful treatment outcome.
- Inhibin B. The amount of this hormone is determined on the third DC and allows you to predict the result of ovulation stimulation. A small level of inhibin B provokes an earlier increase in FSH content and an insufficient response to stimulation.
- Anti-Mullerian hormone (AMH). If its quantity is below normal, this means that the patient has few eggs left and the response to stimulation will be insufficient. The AMH level must be determined before IVF (values from 1.2 to 5 ng/ml are considered normal). The level of the hormone in the blood below 0.8 ng/ml indicates a small chance of a successful pregnancy, but the possibility of conceiving a child using a donor egg remains.
Ultrasound criteria for ovarian reserve. Click to enlarge
Ovarian reserve after 40 years
For women over 40 years of age, it is very important to determine the ovarian reserve before undergoing an IVF cycle.
The in vitro fertilization method is considered a more effective means of enabling women at this age to become pregnant compared to other methods of artificial conception. But a patient aged 40 years or more creates a risk of miscarriage and exposes her child to chromosomal diseases. To prevent such cases, it is better to use a donor egg in women under the age of 40. This method increases the likelihood of a normal pregnancy (60-70%).
Dynamic studies and ultrasound to assess follicular reserve
Description of dynamic studies:
- CC test, which measures the amount of FSH on the third or tenth DC, after taking 100 mg of clomiphene citrate, from the fifth to the ninth DC. An excessive amount of FSH at 10 DC indicates a negative test and most likely indicates a reduction in follicular reserve.
- A test using Gn-RH agonists, in which estradiol is determined on the second DC, and then on the third day after the administration of Gn-RH agonists, or an increase in FSH is detected two hours after the injection of the drug indicated above. An increase in the amount of estradiol in response to an increase in FSH levels caused by the administration of Ang-RH makes it possible to predict the results of stimulation of superovulation.
Purposes of ultrasound:
- Assessment of the volume of the ovary and the number of antral follicles (as the body ages, a decrease in the size of the appendages is observed) by 2-3 DC. Ovarian aging, which sometimes occurs before FSH levels rise, is indicated by a small number of antral follicles and small ovarian volume.
- Analysis of blood flow in stromal arteries. There is a direct relationship between the speed of blood flow and the number of follicles obtained as a result of IVF.
Endometriosis is one of the five most common gynecological diseases after benign diseases of the cervix, menstrual disorders, inflammatory diseases of the urogenital tract and infertility [1]. From 2 to 10% (about 176 million) of women of reproductive age worldwide have this pathology [2, 3]. According to the Federal State Statistics Service, the incidence of endometriosis in Russia over the past 10 years has increased by 72.9% [1]. The incidence of endometrioid ovarian cysts (ECOC) is 17-44% among women with external genital endometriosis [4]. Endometriosis as a cause of infertility ranks second after inflammatory diseases of the uterus and appendages. Up to 40% of ECO patients face infertility problems [5]. Despite numerous studies, the precise mechanisms by which endometriosis affects fertility are not fully understood [6, 7]. Currently, infertility in patients with ECO, along with other significant reasons, is associated with a decrease in the ovarian reserve [8]. Ovarian reserve is understood as the functional reserve of the ovary, which determines the ability of the latter to develop a healthy follicle with a full-fledged egg and an adequate response to ovarian stimulation. Ovarian reserve reflects the number of follicles located in the ovaries (primordial pool and growing follicles) and depends on physiological and pathological factors. One of the main natural factors that leads to a decrease in ovarian reserve is a woman’s age [9]. Belonging to a particular ethnic group can also affect ovarian reserve. Studies [10-12] found that women from India, Southeast Asia, the Caribbean, China and Latin America had lower ovarian reserve compared to age-matched Caucasian and Caucasian women. Pathological factors leading to a decrease in ovarian reserve include: ECO, infections of the pelvic organs (in particular, chlamydia, genital tuberculosis [13]), surgical interventions on the ovaries, chemotherapy and radiation exposure to the pelvic organs [14], excess body weight [15] and smoking [16]. Intoxication with various chemicals that are used in industry, agriculture as pesticides, herbicides, solvents, industrial waste (heavy metals and chemical synthesis products) can also lead to a decrease in ovarian reserve [17, 18]. There are many mechanisms that lead to impaired ovarian reserve in ECO. Back in 1957, P. Hughesdon et al. [19] described that the ovarian cortical tissue located directly next to the EOC showed signs of metaplasia and disorganization. The barrier separating the ovarian tissue from the cyst is composed of fibrous tissue. Recent studies have shown that endometriotic fluid contains a number of toxic substances, such as proinflammatory cytokines and reactive oxygen species [20, 21]. These substances cause fibrosis in the ovarian tissues surrounding the cyst and a decrease in the number of stromal cells in the ovarian cortex. Subsequently, fibrosis, together with reactive oxygen species, leads to a decrease in angiogenesis and a decrease in capillary density in ovarian tissues [22]. Reduced vascularization in the ovarian cortex in EOC can lead to a deterioration in the blood supply to the follicles and their loss. In the cortical layer of the ovaries with endometriosis, accelerated growth of follicles occurs, followed by depletion and a decrease in their number. This burnout theory was proposed by M. Dolmans et al. [23] in 2007. This theory was confirmed in studies by M. Kitajiama et al. [24]. They revealed a significant increase in the percentage of primary follicles in the cortex of the ovary with EOC compared to the intact ovary. These studies found that in ovaries with endometrioid cysts, early follicular development, an increase in the number of atretic follicles, dysregulation of follicle selection, and a decrease in the pool of early follicles may be activated, resulting in the depletion of the pool of primordial follicles. A group of researchers also suggested that inflammation and fibrosis with reduced vascularization and increased oxidative stress, which are observed in the ovarian cortex with endometrioid cysts, lead to impaired folliculogenesis.
In the works of L.V. Adamyan et al. [25, 27, 31, 34] showed a violation of ovarian folliculogenesis in women with endometriosis, characterized by a decrease in the total number of follicles of all stages of development. In patients with ECO, a sharp decrease in the number of primordial follicles and growing follicles was revealed. Moreover, the degree of reduction in the number of follicles in the ovaries of women with endometriosis depends on the stage of spread of the pathological process and the age of the patient. Dystrophic processes in granulosa cells of follicles, changes in the composition of follicular fluid, an increased apoptotic index of granulosa cells, and oocyte degeneration are considered as possible causes of infertility in ECO [27]. Endometrioid cells secrete exceptionally high levels of cytokines and chemokines. Cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor, and chemokines such as IL-8, monocyte chemoattractant protein (MCP)-1, can cause activation of granulosa cells and premature development of follicles with a subsequent decrease in their number [ 28]. Directly or indirectly, these substances can lead to the activation of a cascade of signaling molecules in follicular cells - cyclic adenosine monophosphate protein kinase (cAMP/PKA), tyrosine kinase/signal transducer and activator of transcription (JAK/STAT), nuclear factor kappa B (NF-κB), phosphatidylinositol -3-kinase (PI3K) and intracellular calcium, which leads to premature activation of follicular growth. Endometriotic tissue produces abundant prostaglandin E2 (PGE2), which through adenosine monophosphate protein kinase-α (cAMP/PKA) activates the mitogenic protein kinase pathway (MAPKs), protein 38 (p38), in a number of cell types, including granulosa cells and theca cells in follicles. It can be assumed that PGE2 and other eicosanoids produced in the inflammatory environment surrounding the endometrioid cyst may influence the rate of follicular activation and atresia [29].
Most authors [30] note lower results of ART in women with genital endometriosis in comparison with other factors of infertility (tubal-peritoneal, male, etc.). At the same time, they indicate worse quality of oocytes, a decrease in the frequency of their fertilization and subsequent fragmentation of embryos, as well as a decrease in the frequency of implantation even with “minor” forms of external genital endometriosis. M. Hull et al. note that in patients with ovarian endometriosis, the processes of fertilization of oocytes and fragmentation of the resulting embryos in
vitro
.
According to A. Pellicer et al., a study of embryos at early stages of development showed a relatively low number of blastomeres in embryos from patients with endometriosis compared to controls, as well as an increase in the index of embryos that stopped developing under conditions of fertilization and preimplantation development in vitro
. There is evidence in the literature of a low ability to implant embryos in women with endometriosis-associated infertility. It is assumed that the cause of failures in infertility treatment using reproductive technologies is defective eggs, the development of which occurred under suboptimal conditions. In the works of L.V. Adamyan et al. [31] discovered not only quantitative, but also “qualitative” disorders of folliculogenesis in patients with ovarian endometriosis. Thus, a histological study of fragments of ovaries with endometrioid cysts revealed a large number of follicles with various signs of degeneration (changes in shape and blurred boundaries of the follicle, oocyte and its nucleus, follicular cells; partially decondensed chromatin localized throughout the nucleus; thickening, stratification, hyalinosis of the basement membrane follicle; formation of intercellular spaces; unclear boundary between layers of tissue - thecae internal and external; follicular fluid of different optical densities, unevenly stained with eosin; large vacuoles in the ooplasm; disorganization of granulosa cells; change in the width of the transparent membrane). Molecular biological approaches have made it possible to identify genetic markers indicating a possible predisposition to endometriosis. The study of polymorphism in the genes for xenobiotic detoxification and estrogen metabolism (cytochrome P450 and glutathione-S-transferase) made it possible to detect mutant alleles of these genes (GSTM 1 0/0, GSTT 1 0/0) in patients with endometriosis in combination with a slow form of N-acetyltransferase. If a mutation in the erythrocyte esterase D*7 gene (ESD*7) or serum esterase D*5 gene (ESD*5) is detected in women, an increased predisposition to ovarian endometriosis in combination with peritoneal endometriosis (ESD*7) and to the formation of endometrioid cysts can be assumed. ovaries (ESD*5) [32, 33]. Thus, the expression of genes leading to the development of endometriosis may influence follicle formation in the early stages of ovarian formation. A defect in folliculogenesis that occurs in the embryonic period can subsequently manifest itself both in the formation of an initially low number of follicles and in impaired function of follicular cells [34]. These studies support the theory that EOCs may cause ovarian damage prior to surgery. When performing surgical treatment for ECO, an important aspect is to study the state of the ovarian reserve before surgery. This will make it possible to assess the degree of damage to ovarian tissue before surgery, develop individual tactics for managing women with EOC, and restore reproductive function in these patients.
There are the following criteria on the basis of which the state of the ovarian reserve can be assessed: 1) the woman’s age; 2) concentration of follicle-stimulating hormone (FSH), inhibin B, estradiol (E2), anti-Mullerian hormone; 3) ultrasound characteristics of the ovaries: number of antral follicles, ovarian volume, indicators of intraovarian blood flow. Studies conducted in recent years [35, 36] have shown that the rate of follicle disappearance doubles when the primordial pool decreases to 25,000 follicles, which normally corresponds to the age of 37.5 years. This age is defined as critical, after which the ovarian reserve sharply decreases. Measurement of FSH levels on days 2–3 of the menstrual cycle (basal level) was proposed as the first hormonal test to determine ovarian reserve. But in different menstrual cycles, FSH levels can fluctuate from high to low values. This is alarming regarding a decrease in the patient’s ovarian reserve, but is not an absolute marker of the state of ovarian function [37]. Subsequent studies [38] revealed that inhibin B, a specific protein similar in structure to transforming growth factor, plays a leading role in regulating FSH levels at the beginning of the menstrual cycle. Inhibin B is a heterodimeric glycoprotein consisting of two subunits - α and β, with a molecular weight of about 32 kDa each. This protein is produced by Sertoli cells of the ovary and plays an important role in the regulation of folliculogenesis. Granulosa cells of the small antral follicles of the ovary produce high concentrations of inhibin B, and this is what determines the basal level of FSH at the beginning of the menstrual cycle [39]. It was found that lower levels of inhibin B at the beginning of the menstrual cycle reflect a reduced population of small antral follicles and cause a sharper increase in FSH concentrations, which ultimately leads to rapid growth of the dominant follicle and earlier ovulation. Similar mechanisms occur with reduced ovarian reserve [38].
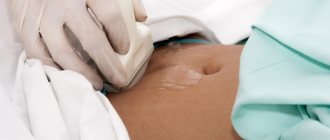
Ultrasound plays an important role in assessing ovarian reserve. Currently, the volume of the ovaries is determined on the 2-5th day of the cycle and is calculated based on three measurements made in two perpendicular planes, according to the formula: V
=0.5236×
L
×
W
×
T
, where
L
is the length,
W
is the width,
T
is the thickness of the ovary. It has been established that an ovarian volume of less than 3 cm3 indicates a poor prognosis regarding ovarian reserve [40]. Counting the number of antral follicles is a more accurate method of assessing ovarian reserve. According to a number of researchers [39, 40], three groups of a woman’s ovaries can be distinguished depending on this indicator: inactive (less than 5 follicles), normal (5-15) and polycystic (more than 15). Adequate blood supply to the ovaries is essential for their normal functioning.
One of the main Doppler criteria for predicting the response of the ovaries to stimulation is the peak systolic velocity (PSV) of blood flow in the vessels of the ovarian stroma. With an insufficient response to stimulation, the average PSS of the stromal arteries on days 2-3 of the cycle was 2 times lower than with a normal response. An increase in the pulsatility index (PI) and resistance index (RI) of the perifollicular vessels of the ovaries of patients with an insufficient response to stimulation and normal FSH levels was noted [41]. Indicators of reduced ovarian reserve and insufficient response include low PSS (less than 10 cm/s), high values of the pulsatility index and resistance index.
Determination of anti-Mullerian hormone (AMH) levels is currently considered the most accurate quantitative method for assessing ovarian reserve [42]. AMH is a member of the transforming growth factor β family and plays a critical role in the embryogenesis of male mammals. AMH is produced by Sertoli cells, which causes regression of the Müllerian duct organs (fallopian tubes, uterus and upper vagina). In the ovaries, AMH is produced from the prenatal period (from the 32nd week of embryonic development) to menopause and is responsible for the transition of resting primordial follicles into the active growth phase, as well as for the selection of FSH-sensitive follicles at the early antral stage. This hormone is secreted by granulosa cells of growing follicles - from the preantral stage to the size of antral follicles 6-8 mm in diameter. After the follicles reach 8 mm or more, the AMH level sharply decreases, aromatase activity increases and, accordingly, estradiol production increases. AMH characterizes follicles at the stage preceding the hormonally-dependent period of follicular growth and protects the granulosa of growing follicles from the excessive mitogenic influence of FSH. This allows us to obtain information about the deeper processes of folliculogenesis and estimate the number of growing follicles at the hormone-sensitive stage of their growth. Standard values for AMH are considered to be 1.0–2.5 ng/ml. A decrease in AMH levels indicates a decrease in ovarian reserve.
In 2011, the European Society of Human Reproduction and Embryology (ESHRE) [43] created a working group that approved standard criteria for defining “reduced ovarian reserve.” These criteria were called the “Bologna criteria”. According to the ESHRE consensus, the basis for stating a reduced ovarian reserve should be two of the following three criteria: the patient’s age is 40 years or older; poor ovarian response to ovulation stimulation (3 oocytes or less); low AMH levels (less than 0.5-1.1 ng/ml) and a reduced number of antral follicles (less than 5-7 according to ultrasound). Thus, a comprehensive study of ovarian reserve indicators in women of reproductive age with ECO at the stage before surgical treatment will create a holistic picture of the degree of damage to ovarian tissue and develop a ranked approach to the treatment of these patients. The opinion of researchers regarding the influence of endometrioid formations of the ovaries on changes in AMH levels is ambiguous. In studies [44], in most patients with ovarian endometriosis, AMH values did not differ from those in the comparison group. Similar results were obtained by the authors in [45]. Other researchers [46] found low levels of AMH in patients with ovarian endometriosis of various stages of spread. Y. Hwu et al. [47], assessing the concentration of AMH, obtained significantly lower AMH values in bilateral endometriotic ovarian formations compared to unilateral ones. These studies confirm the need to find more accurate markers of ovarian reserve.
Currently, much attention is being paid to identifying microRNAs as a new non-invasive marker of endometriosis, as well as a marker of decreased ovarian reserve.
MicroRNAs (miRNAs) are small non-coding RNA molecules with an average length of 22-24 nucleotides. They were discovered in 1993 by Ambros et al. MicroRNAs take part in the transcriptional and posttranscriptional regulation of gene expression through RNA interference [48]. Scientists have established a connection between microRNA and the proliferation of endometrioid cells. A group of researchers led by L. Ramón [49] analyzed several miRNAs associated with angiogenesis and angiogenic factors, such as vascular endothelial growth factor-A (VEGF-A) and thrombospondin-1 (TSP-1), in endometriotic lesions (ECLs). , peritoneal lesions and rectovaginal nodes) and in the eutopic endometrium of women with endometriosis. The authors used the latest methods, real-time polymerase chain reaction (PCR) (TaqMan), to evaluate the expression of miRNAs (miR-15b, -16, -17−5p, -20a, -21, -125a, -221 and -222) , while the expression of VEGF-A and TSP-1 mRNA was studied using real-time PCR method; The content of VEGF-A and TSP-1 proteins was determined using a quantitative ELISA method. The study included 58 women with endometriosis and 38 women in the control group. According to the results of the study, significantly lower levels of VEGF-A mRNA were found in paired EOC tissue samples ( p
=0.02) and the expression of the protein itself (
p
=0.002) than in the eutopic endometrium, and higher expression of miR-125a (
p
=0.003) and miR-222 (
p
<0.001).
However, ovarian endometrioma tissue showed significantly higher expression of the angiogenic inhibitor TSP-1 and lower expression of miR-17−5p than eutopic endometrium ( p
< 0.001).
In addition, significant inverse correlations were found between the expression of miR-222 and the content of VEGF-A protein ( r
=–0.267;
p
= 0.018) and between miR-17−5p and the content of TSP-1 protein (
r
=–0.260;
p
= 0.022 ).
Peritoneal lesions showed a significant increase in VEGF-A compared to ovarian endometriomas ( p
< 0.01). The authors [49] concluded that the expression levels of miRNAs associated with angiogenesis differed between eutopic endometrium and ovarian endometrioma tissue. These differences may influence the expression of angiogenic factors that play an important role in the pathogenesis and pathophysiology of endometriosis.
The authors of the following study [50] were the first to conduct transcriptome-microRNAome analysis in endometrioma tissue and eutopic endometrium using the latest sequencing technology. The authors were able to reproduce more than 54 million independent microRNAs in 19 clinical samples, while the expression of some microRNAs was increased, while the expression of others was significantly reduced. We identified 10 microRNAs whose expression was increased (miR-202, 193a-3p, 29c, 708, 509−3-5p, 574−3p, 193a-5p, 485−3p, 100, and 720), and 12 micro-RNAs. RNAs whose expression was significantly reduced (miR-504, 141, 429, 203, 10a, 200b, 873, 200c, 200a, 449b, 375, and 34c5p) in EOC compared to eutopic endometrium. The functional significance of these differences was tested in a study performed on endometrial stromal fibroblasts in
vitro
, which showed that in the extracellular matrix, miR-29c expression was decreased, while miR-29c expression was significantly increased. This new approach confirms the contribution of microRNAs to the pathophysiology of endometriosis. Numerous microRNAs expressed in the ovary regulate follicular growth, atresia, ovulation and steroidogenesis and play an important role in ovarian dysfunction [51]. Scientists [52] have found that microRNAs are involved in the proliferation and apoptosis of granulosa cells. Thus, recent studies [53] found that microRNA-143 suppresses the formation of primordial follicles by reducing the expression of cyclin-dependent kinases 4 and 6 and cyclins B1, D2 and E2 in pregranulosa cells. In addition, microRNA-181a suppresses the proliferation of granulosa cells in mouse ovaries by activating the IIA receptor [54], microRNA-26b stimulates apoptosis of ovarian granulosa cells [55]. In addition, a decrease in the plasma level of microRNA-22−3p significantly correlates with a decrease in ovarian reserve [56]. Overexpression of microRNA-23a enhances apoptosis in granulosa cells [52].
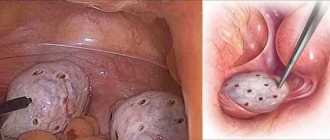
Currently, the basis for diagnosis and treatment of various forms of endometriosis is surgical intervention. According to prospective cohort studies [57, 58], among infertile women with moderate and severe stages of endometriosis followed by laparoscopy and removal of endometrioid lesions, spontaneous pregnancy occurs in 52-69% of women. At the same time, the question remains open regarding the effect of surgical treatment of women with ECO on the ovarian reserve. Ovarian resection may inadvertently damage the ovarian reserve. M. Coccia et al. [59] conducted a prospective cohort study involving 302 patients who underwent laparoscopy to remove EC from March 1993 to November 2007. It was found that women who underwent bilateral cystectomy entered menopause 5 years earlier than patients with unilateral ovarian resection. In a study by S. Takae et al. [60] confirmed the negative impact of ovarian resection for endometrioid cysts on the ovarian reserve, which is a risk factor for premature ovarian failure. These studies [61] confirm the need for long-term monitoring of the ovarian reserve of patients who have undergone ovarian resection for ECO. The effect of ovarian resection for endometrioid cysts on ovarian reserve is determined by the level of AMH in the blood serum after surgery. E. Somigliana et al. (2012) noted a decrease in ovarian reserve up to 53% after laparoscopic removal of the EC. According to a number of researchers, a possible reason for the decrease in ovarian reserve is the removal of healthy ovarian tissue along with endometrioid formation. Many investigators have reported the presence of primordial follicles adjacent to the cyst wall in a significant number of endometriosis specimens (J. Shi J, 2011; D. Romualdi, 2011; E. Dogan, 2011; H. Oh et al.). Thus, M. Kitajima et al. [62] in their studies confirmed the presence of normal ovarian tissue in an enucleated endometrioid cyst, resulting in a decrease in AMH levels by 42% of the initial level.
Thus, when treating patients of reproductive age with ECO, the most important issue remains the preservation of fertility. When removing an endometrioid cyst, all principles of microsurgical operation should be observed, taking into account the characteristics of the gentle effect of the instrument, energy modes (electric, laser, cryo, plasma, ultrasound, etc.) on ovarian tissue (enucleation of only affected areas) for maximum preservation of ovarian tissue reserve [63]. Until now, there is no clear answer to the question of the advisability of removing the EOC capsule when it is small in size (up to 30 mm). According to the 2013 Global Consensus on Contemporary Management of Endometriosis [64], laparoscopic excision should be performed for EEC larger than 4 cm in diameter. As mentioned above, the presence of ECO leads to the early development of follicles and their atresia, the regulation of follicle selection is disrupted, as a result of which the pool of primordial follicles is exhausted, and the quality of the remaining primordial follicles is low. Thus, the presence of endometrioid cysts and associated structural changes in tissue in the ovarian cortex may be the cause of a decrease in ovarian reserve. A number of scientists [65] have established that these processes are also observed at the early stage of development of ECO, ranging from 1 to 4 cm. These results are an argument against wait-and-see tactics in the case of ECO. Early diagnosis and surgery may be beneficial for women with endometriosis to preserve ovarian function.
It is also controversial whether the damage to ovarian tissue during surgical removal of endometrioid cysts is more serious than damage to the ovarian cortex resulting from the toxic effects of the endometrioid cyst. According to a number of studies [66], a statistically significant correlation was found between the rate of decrease in the level of AMH in the blood serum and the diameter of the endometrioid cyst and the preoperative level of serum AMH. The rate of decrease in AMH levels after surgery was significantly higher in the subgroup of women with endometrioid cysts with a diameter of more than 7 cm than in the subgroup of women with ECO with a diameter of less than 7 cm. The decrease in ovarian reserve is inversely related to the preoperative level of AMH in the blood serum. The age of patients is a factor that negatively affects ovarian reserve [66]. In studies by T. Lind et al. [67] showed a pronounced decrease in AMH levels in bilateral EOCs than in unilateral ovarian lesions. A significant decrease in AMH levels was observed in women with initially high and average levels - by 23 and 43% after 3 and 6 months, respectively. Women with low baseline AMH levels had minimal or no changes after surgery. The postoperative decrease in AMH levels was greater at 3 months in women with significant intraoperative bleeding (500 and 700 ml). Patient age, cyst size, and duration of surgery were not predictors of postoperative reduction in AMH levels in this study.
The question of the choice of energy during surgical removal of EOC from the standpoint of preserving the ovarian reserve remains controversial. It is known that there is a higher risk of tissue damage when using monopolar coagulation. This is due to the fact that monopolar coagulation has a delayed coagulating effect on ovarian tissue and is not always predictable with respect to the depth of effect [68]. The difference between bipolar electrosurgery is the locality of the impact, which allows you to control the boundaries of destruction. Thus, in a study by R. Horace et al. [69], which included 22 women with a unilateral endometrioid cyst with a diameter of more than 30 mm, without previous surgical intervention and without a history of pregnancy, it was found that before surgery (removal of EOC using plasma energy) the average AMH values were 3. 9±2.6 ng/ml, 3 months after surgery - 2.3±1.1 ng/ml and at the end of the observation period 3.1±2.2 ng/ml. An AMH level of more than 2 ng/ml was observed in 71% of cases in the postoperative period (at the end of observation) versus 76% of cases before surgery. These studies confirm that bipolar coagulation, in contrast to plasma energy and CO2 laser, has a more pronounced negative effect on the state of ovarian reserve. The most gentle effect on ovarian tissue is the use of a CO2 laser. M.F. Doffman et al. (2013) in their studies showed the most gentle effect of argon plasma coagulation on ovarian tissue, in contrast to bipolar coagulation. A.I. Davydov et al. (2012) believe that the Plasma-Jet system is distinguished not only by minimal damaging effects on ovarian tissue, but also by complete destruction of the endometrioid lining of the endometrioid cyst. A number of scientists point out the advantage of using suture technology for the purpose of hemostasis. In studies by A. Ebert et al. [70] showed the high efficiency of using a gelatin-thrombin matrix. Thus, due to conflicting opinions about the safety of the ovarian reserve in ECO, the lack of a unified view on the effect of various surgical energies on ovarian tissue, as well as in some cases the small number and heterogeneity of groups of patients included in the study, there is a need to continue research on the study of ovarian reserve in women with ECO using modern molecular genetic methods.
Manuscript submission date:
10.02.2017
Sources of financing:
none.
Financial conflict of interest:
absent.
Other forms of conflict of interest:
none.