Without any doubt, we can say that infertility is a huge problem that has taken many young couples by surprise. Judging by annual statistics, recently the number of women suffering from this disease has been increasing very quickly. This phenomenon often occurs due to pathologies in the endometrium, which, by the way, could have been eliminated even before pregnancy.
However, the chance of a full recovery directly depends on when the problem was identified and when it was treated. To determine possible causes, a considerable number of methods can be used, one of which is endometrial immunohistochemistry. This is a rather complex and, in turn, specific range of tests, which is prescribed exclusively by the attending physician.
What it is?
Immunohistochemistry of the endometrium is a special and rather difficult study of the endometrium. This analysis allows for extensive examination and diagnosis of the uterine mucosa. Judging by the name, it becomes clear that IHC includes both histological and immunological studies. The former are responsible for studying the composition of tissues, and the latter for searching for pathogens and antibodies. During the study, it will be possible to establish the presence of cells that do not allow a woman to become pregnant. During this procedure, their number will also be determined.
With the help of such an analysis, it will be possible to assess how sensitive the endometrial receptors are to natural hormonal stimulation during the period when ovulation occurs, as well as during active hormone therapy before in vitro fertilization. This is done due to the fact that the following problem often arises: the woman’s health is normal, her hormonal levels and endometrium are the same, antisperm antibodies have not been found, but despite this, she cannot get pregnant. The main reason for this phenomenon is the following fact: the endometrium refuses to perceive hormonal effects, and therefore does not renew itself and does not thicken.
Why is IHC analysis used in oncology?
Immunohistochemistry helps solve the following problems:
- Clarify the diagnosis, type and subtype of malignant tumor, if cytological and histological examination does not allow this. For example, there are about 70 types of lymphomas and leukemias. They are most often diagnosed using immunohistochemistry. Sometimes additional research is required.
- To identify the nature and localization of the main tumor, if only distant metastases were detected and their origin is unclear.
- Select the optimal treatment regimen. Using immunohistochemical analysis, it is possible to assess the sensitivity of a malignant tumor to certain drugs and to detect “molecular targets” for antitumor drugs.
- Assess the degree of malignancy of tumor cells, prognosis.
- Differential diagnosis: the study helps to distinguish malignant tumors from benign neoplasms and non-tumor processes.
- Diagnose multiple tumors. Cancer immunohistochemistry is used when a patient is suspected of having two or more different types of malignant tumors at the same time.
What it detects and when it is prescribed
As mentioned above, the purpose of IHC is to diagnose infertility. Thus, let us consider the most common cases of prescribing immunohistochemical analysis:
- In vitro fertilization was unsuccessful;
- The patient constantly experiences miscarriages in early pregnancy;
- The woman has symptoms of infertility, but the diagnosis has not yet been confirmed;
- Diagnosed with infertility.
In addition, as experience shows, with such a study, in addition to the cause of infertility, a different type of endometrium can be identified. In this regard, IHC is often prescribed in cases where it is necessary to diagnose chronic sluggish endometritis, which cannot be determined by other methods. Also during the study the following diagnoses can be made:
- Incomplete transformation of the endometrium;
- Endometritis;
- Disturbance and desynchronization of the phases of its development;
- Hyperplasia.
In approximately 70-75% of cases, it is one of the above reasons that leads to infertility. It is worth noting that all these diseases are quite easy to treat if they are identified in the early stages.
IHC mainly examines a biopsy of the uterine lining. This material is obtained using a special aspiration curette - pipel. The process is painless and does not require the use of anesthesia.
Upon completion of the study, the doctor calculates the duration of the implantation window - a certain period of time during which the greatest correspondence between the endometrium and the embryo is observed. During this period, the presence of pinopodia is checked. Normally, the implantation window begins to be recorded from the 20th to the 24th day of the menstrual cycle.
Cost of analysis at the Medicine 24/7 clinic
At the Medicine 24/7 clinic, immunohistochemical tests for cancer and a number of other diseases are performed using fluorescence in situ hybridization. Antibodies attached to antigens give off a glow when examined under a microscope. Prices for immunohistochemistry in our clinic are presented below.
The material was prepared by the deputy chief physician for medical work of the Medicine 24/7 clinic, candidate of medical sciences Sergeev Petr Sergeevich.
Sources:
- Beysenaeva A. R. The role of immunohistochemical research in oncology // Medicine and ecology. 2015. No. 2 (75).
- Nepomnyashchikh L. M., Lushnikova E. L., Verzhbitskaya N. E., Mitelman Yu. M., Zhuk A. G. Immunohistochemical studies in oncomorphology // Siberian Journal of Oncology. 2006. No. 3.
- Zhuk A.G., Ivshina Yu.A., Mitelman Yu.M. Immunohistochemical study of hyperplastic processes and breast cancer // Siberian Journal of Oncology. 2006. No. 3.
Using immunohistochemical research, the following is performed:
- determination of the type and subtype of tumor;
- determination of the prevalence of cancer;
- when examining metastases, their source is determined;
- assessment of the effectiveness of cancer treatment;
- determining the degree of malignancy of the tumor;
- The proliferative activity of tumors is determined (at what speed they grow).
Contraindications
Immunohistochemical examination of the endometrium has no special contraindications. The only case when it is impossible to perform this analysis is the impossibility of collecting tissue samples needed for research. However, it is worth noting the indications for this procedure:
- Repeated failed IVF attempts;
- Early pregnancy loss;
- Suspicion of a malignant neoplasm;
- Study of infertility and identification of its causes.
It is worth noting that almost any tissue can be examined using IHC. Among the above indications, the most important is the suspicion of a malignant neoplasm.
How is an immunohistochemical study performed?
The research itself consists of four stages:
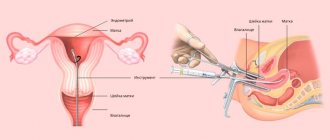
- Pre-laboratory stage, which consists of obtaining an adequate tissue sample for analysis. Tissue for examination can be obtained using incisional or excisional biopsy, punch-biopsy (using forceps), or during endoscopic surgery. The procedure for obtaining a biopsy, as well as preparation for it, are determined by the type and location of the tumor. The resulting tissue is placed in a 10% formaldehyde solution and sent to the laboratory.
- Preparation, during which the biopsy sample is processed followed by its initial study. At the same stage, the thinnest sections are prepared from a piece of tissue.
- Staining of sections with immunohistochemical preparations, which are a solution of specific antibodies. Depending on how many different types of antibodies I use, there are small and large panels of research. The small panel includes up to 5 antibodies, the large panel – from 6 to several dozen. The number of markers determined depends on the intended diagnosis.
- Research and analysis of painted samples, after which a conclusion is made.
Proper preparation
Since the collection of the material needed for research is carried out using the method of conventional curettage, you need to understand that it needs to be carried out only during the period when the endometrium is ready for this. Because of this, the doctor is forced to schedule tests on a specific day. This procedure is strictly not recommended on any other day. In most cases, the material can be collected on days 5-7 of the cycle to diagnose inflammatory processes and growths. If an assessment of receptor function is required, then the procedure is performed on days 20-24.
In addition, there is a small list of rules that must be followed:
- Do not take hormonal medications for a week before the test;
- Do not take blood thinners to avoid bleeding after the biopsy procedure;
- Follow all hygiene rules on the day the IHC is performed.
How is the analysis carried out?
In order to conduct a study for various tumor markers, the doctor must perform a biopsy - obtain a piece of tissue from a malignant tumor and send it to the laboratory. There, the material is prepared in a special way (using paraffinization or freezing), treated with appropriate antibodies and examined under a microscope. Immunohistochemistry of tumors allows not only to identify the substance of interest, but also to assess its localization in the tissue and judge its quantity.
After the study is completed, the attending physician receives a conclusion from the laboratory. The timing of the analysis is established by paragraph 24 of Order of the Ministry of Health of Russia No. 179n dated March 24, 2021. If tumor tissue is examined for 5 different markers or a smaller amount, the result should be ready within one week, if for a larger amount - within 15 days.
Progress of the procedure
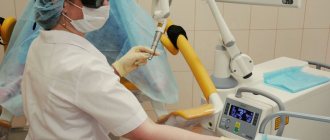
Immunohistochemical analysis of the endometrium is carried out as follows:
- An anesthetic is administered;
- Installation of special expanders;
- Preventive measures;
- Insertion of a hysteroscope;
- Curettage procedure;
- Removing the drug;
- Cauterization of damaged vessels;
- Removing equipment;
- Repeated scanning of each organ;
- Removing the expanders.
After all these procedures are completed, the patient is transferred to a separate room, where she will remain until she recovers from anesthesia. After this, she can already leave the hospital. Results will be available within a few days.
Positive and negative sides
- A minimum of biomaterial and reagents are required;
- Detects cancer at an early stage;
- Quick results;
- Accuracy and reliability.
An additional advantage is the possibility of three-dimensional modeling. The antibody response is visible after three hours. The final conclusion is given in 7-10 days. After this time, the type of cancer and stage of the disease, as well as its primary focus, will be accurately determined. Changes in the color of the markers usually do not cause discrepancies when making a diagnosis. A transcript of the results is written on the final form.
Reliability depends on the method of staining the sample with reagents. This is done automatically and manually. With the manual method, the human factor and laboratory technician errors cannot be excluded. The attending physician will tell you which laboratory to contact. The patient can independently inquire about how the analysis is carried out in a particular institution. To ensure the reliability of the result, it can be repeated or in two laboratories.
The resulting error is the fault of the patient. Before the biopsy, you should avoid hormonal drugs and other medications, exercise, and adhere to a diet. External factors influence results.
Decoding
Decoding is performed only by a doctor due to the fact that there is no standard common to all. It mainly depends on the physiological age of the woman, her hormonal levels and other factors. The situation with endometritis is simpler.
The specialist uses the obtained test results to determine the shift of the implantation window. In addition, the condition of the endometrium at a certain point in the cycle is checked. The results of the biopsy analysis will be able to indicate with 100% accuracy whether the patient has an inflammatory process in the uterine cavity.
Additional Information
Literature:
- Draft order of the Ministry of Health of Russia dated November 21, 2012 “On approval of the Procedure for the provision of medical care in the profile of “pathological anatomy”
- Immunohistochemical methods: a manual. Per. from English edited by G.A. Frank and P.G. Malkova // M., 2011, – 224 p.
| service price | from 2750 rub. |
available in 199 clinics Moscow
- Tumor marker alpha-fetoprotein (AFP), (blood)
- Tumor marker SA - 19-9, (blood)
- Immunochemistry of blood and urine (complex)
- Histological examination of biopsy material
- Medical and speech therapy research
- Ultrasound of the kidneys and adrenal glands
Invalid phone number format.
Your request for a call back has been accepted.
Dear user, we sincerely apologize, but the version for the visually impaired is temporarily unavailable for technical reasons.
1.1. These Regulations (hereinafter referred to as the “Regulations”) were adopted by the Limited Liability Company “Medoblako” (hereinafter referred to as the Operator) and are intended to determine the basis of the Operator’s activities to ensure the safety and confidentiality of personal data of citizens in accordance with the requirements of the current legislation of the Russian Federation, as well as for the purpose of regulating the procedure for working with personal data in the Organization.
1.2. The Regulations were developed in accordance with the Operator’s Charter, Federal Law No. 152-FZ dated July 27, 2006 “On Personal Data”, Federal Law No. 323-FZ dated November 21, 2011 “On the Protection of the Health of Citizens in the Russian Federation”, legislative acts of the Russian Federation.
1.3. This provision is mandatory for compliance by all employees of the Operator.
1.4. The Regulations come into force from the moment of its approval by the order of the General Director of the Operator and are valid until the approval of the new Regulations.
1.5. All changes and additions to the Regulations must be approved by order of the General Director of the Operator.
2.1. Personal data is any information relating to a directly or indirectly identified or identifiable individual (subject of personal data).
2.2. Processing of personal data is any action (operation) or set of actions (operations) performed using automation tools or without the use of such means with personal data, including collection, recording, systematization, accumulation, storage, clarification (updating, changing), extraction, use, transfer (distribution, presentation, access), depersonalization, blocking, deletion, destruction of personal data.
2.3. Automated processing of personal data – processing of personal data using computer technology.
2.4. Personal data operator is a person who, independently or jointly with other persons, organizes and (or) carries out the processing of personal data, as well as determines the purposes of processing personal data, the composition of personal data to be processed, and actions (operations) performed with personal data.
2.5. Submission of personal data – actions aimed at disclosing personal data to a certain person or a certain circle of persons.
2.6. Dissemination of personal data – actions aimed at disclosing personal data to an indefinite number of persons.
2.7. Destruction of personal data – actions as a result of which it becomes impossible to restore the content of personal data in the personal data information system, and (or) as a result of which material media of personal data are destroyed.
2.8. Depersonalization of personal data are actions as a result of which it becomes impossible to determine the ownership of personal data by a specific subject of personal data without the use of additional information.
2.9. Subjects of personal data are the Operator’s employees, employees and representatives of the Operator’s current and potential counterparties, current and potential clients of the Operator, representatives (by force of law and by proxy) of the Operator’s current and potential clients.
3.1. In its activities, the operator ensures compliance with the principles of processing personal data specified in Art. 5 of the Federal Law of July 27, 2006 No. 152-FZ “On Personal Data”.
3.2. The operator collects and further processes personal data for the following purposes:
— maintaining personnel, accounting and tax records;
— actual implementation of the types of activities provided for by the Operator’s constituent document, external and internal control of business processes;
— interaction with counterparties, conducting contractual work within the framework of the emergence, change and termination of legal relations between the Operator and third parties, as well as execution of powers of attorney to represent the interests of the Operator;
— consideration and recording of incoming requests of any nature, provision of information services to clients;
— implementation of remote interaction of the Operator with clients and other persons within the framework of service, information and other services through the use of telephone communications and e-mail;
— organization and implementation of a set of measures aimed at maintaining and (or) restoring the health of clients and including the provision of medical services;
— organization and implementation by the Operator of internal quality control of medical care;
— implementation of remote interaction between the Operator and clients and other persons through the Operator’s website on the Internet.
3.3. The processing of personal data by the Operator includes collection, recording, systematization, accumulation, storage, clarification (updating, changing), extraction, use, transfer (distribution, provision, access), depersonalization, blocking, deletion, destruction of personal data.
3.4. The operator processes personal data using automation tools and without the use of automation tools.
3.5. The operator has established the following conditions for terminating the processing of personal data:
results
In all groups, the histological characteristics of the endometrium corresponded to simple endometrial hyperplasia without atypia.
There was an increase in the number of both glandular and stromal elements. However, there was no noticeable close arrangement of the glands. The glands were round and varied in size.
The results of immunohistochemical examination of the endometrium of all patients are presented in Table. 2.
Table 2. Results of immunohistochemical study of the endometrium in patients of the examined groups Note. * — number of positive cells in the field of view at a magnification of 400; **—proliferation index (ratio of the number of stained nuclei to the total number of nuclei,%); *** — number of stained nuclei of stromal cells in the field of view at a magnification of 400; # is the number of stained nuclei in the field of view at a magnification of 400; ## — number of positive cells.
conclusions
1. Features of changes in the levels of morphofunctional and immunohistochemical markers of the endometrium and indicators of the immune response in women of different age groups with endometrial hyperplasia and infertility were identified.
2. The data obtained on an increase in the expression of the proliferation marker Ki67, a small number of CD56-positive cells, a small number of CD68 macrophages and a decrease in the expression of the VEGF marker allow us to conclude that the prognostically most unfavorable period for a possible pregnancy is the age of a woman over 40 years old.
Discussion
In groups 1 and 2, uneven, low proliferative activity was revealed. There was a tendency toward greater expression of the cell proliferation marker Ki67 (glands) in patients of group 3, which corresponded to the greatest activity of the hyperplastic process in this group and could indicate the presence of an incomplete “implantation window.” In groups 1 and 2, there was a decrease in the expression level of Ki67 (stroma) compared to this indicator in group 3 ( p
<0.05). In patients of older reproductive age, there was an increase in the expression of the proliferation marker Ki67, which may serve as evidence of an increase in the processes of proliferation of endometrial cells and their apoptosis, especially in the superficial layers (Fig. 1).
Rice.
1. High expression of Ki67 in the epithelium of glands and stromal cells in patients of group 3. Immunohistochemical reaction. Uv. 200. When comparing the expression of estrogen (ER) and progesterone (PR) receptors, no significant differences were found between the groups. The study found that the ratio of ER and PR in the proliferation stage was almost equal to 1. In groups 1, 2 and 3, the expression rates of ER and PR were approximately equal ( p
>0.05). Thus, no age-related differences in the expression of ER and PR in the proliferative phase were detected.
In all groups, there was a small number of main immunocompetent endometrial cells (CD56-positive cells) ( p
>0.05), manifested by low cytotoxic activity and a small number of CD68 macrophages in the endometrium, which may adversely affect the implantation process (Fig. 2).
Rice.
2. Weak representation of CD56-positive lymphocytes in the endometrial stroma in patients of the examined groups. Analysis of the concentration of the plasma cell marker CD138 showed that the level of its expression in patients of groups 1 and 2 was low ( p
<0.05), and in the endometrium of patients of group 3 there was no expression of CD138. This indicated minor inflammatory changes in the endometrium in patients of groups 1 and 2 and the absence of inflammatory processes in patients of group 3.
In the 3rd group, VEGF expression manifested itself as a positive reaction in individual stromal cells; there was no expression in the vascular endothelium, while in the 1st and 2nd groups a positive reaction was observed in all stromal cells and in the vascular endothelium. This indicated the greatest decrease in neoangiogenesis processes in group 3, and thus insufficient blood supply, and led to hypoxia due to a decrease in oxygen diffusion. Low VEGF levels contributed to poor vascular formation, which further reduced vascular blood flow in the endometrium.
The expression of CD34 (a marker responsible for the early stages of hematopoiesis) in all groups was manifested by a positive intense reaction in the endothelium of the spiral arteries, numerous capillaries and stromal cells, which indicated that the vessels were formed normally and there was normal proliferation of endothelial cells (Fig. 3).
Rice. 3. Numerous small vessels in the stroma of the hyperplastic endometrium. Immunohistochemical reaction with antibodies to CD34. Uv. 200.
However, in group 2 there were superior indicators for this phase of the menstrual cycle (proliferation), which could indicate that there is an increased process of angiogenesis in the endometrial stroma. Normally, an increase in CD34 expression in the basal layer and stroma of the endometrium was observed in the second phase of the cycle. Thus, in patients of group 2, one could assume a low probability of endothelial cell fibrosis, which did not exclude its presence in patients of groups 1 and 3.